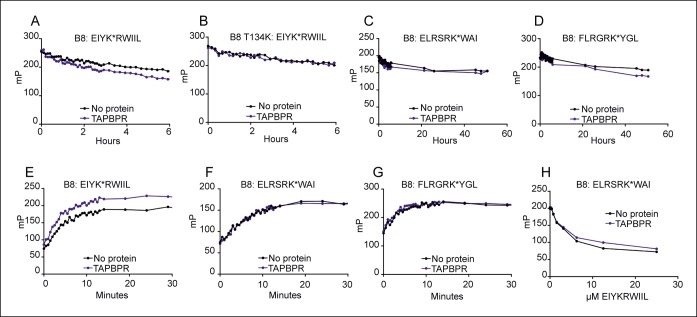
Figure 4. TAPBPR can function as a peptide loading catalyst and peptide editor for HLA-B8.
(A–D) Dissociation, (E–G) association and (H) peptide competition of fluorescent peptides on HLA-B*08:01 in the absence or presence of TAPBPR. 2 μM HLA-B*08:01fos or HLA-B*08:01fos T134K molecules were mixed with 20 μM human β2m and made peptide receptive then loaded with 1 μM (A,B) EIYK*RWIIL (C) ELRSRK*WAI or (D) FLRGRK*YGL. Dissociation was subsequently followed after the addition of a 250 molar excess of (A,B) EIYKRWIIL (C) ELRSRKWAI, or (D) FLRGRKYGL in the absence or presence of 0.75 µM TAPBPR. A total of eight dissociation experiments have been conducted for wild-type HLA B*08:01fos, and the T134K mutant was included in six of these experiments. (E–G) 0.6 μM HLA B*08:01fos molecules were mixed with 6 µM human β2m and made peptide-receptive, then the binding of 1 µM of (E) EIYK*RWIIL, (F) ELRSRK*WAI or (G) FLRGRK*YGL was followed in the absence or presence of 0.175 µM TAPBPR. One of three experiments is shown. (H) 0.6 μM HLA-B*08:01fos molecules were mixed with 0.6 µM human β2m and were made peptide-receptive, then incubated with 0.1 μM high affinity peptide ELRSRK*WAI and various concentrations of the lower affinity competing peptide EIYKRWIIL (0–25 μM) in presence or absence of 0.3 μM TAPBPR. One of six experiments is shown. Fluorescence polarisations measurements were taken after being left at room temperature overnight. While there were slight modifications of experimental conditions between replicate experiments, results consistent with the presented results were observed.