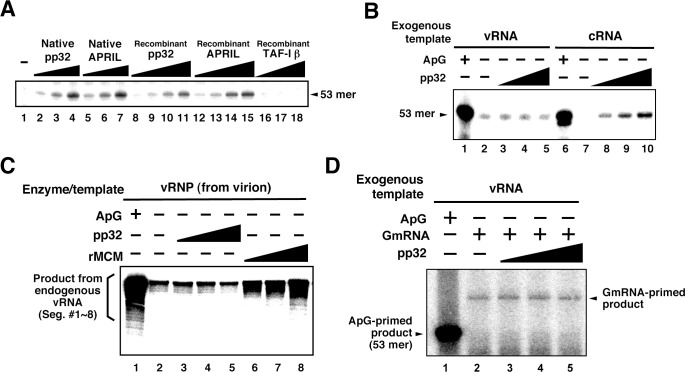
Figure 3. Characterization of influenza virus replication factor-2 (IREF-2) activities in the cell-free system.
(A) Dose response of IREF-2. Native or recombinant IREF-2 proteins were added to the cell-free viral RNA (vRNA) synthesis reaction using micrococcal nuclease-treated vRNP (mnRNP) and the c53 model template in the absence of primer. Native pp32 (lanes 2–4), native APRIL (lanes 5–7), recombinant pp32 (lanes 8–11), and recombinant APRIL (lanes 12–15) were used as follows: 5 ng (lanes 2, 5, 8, and 12), 15 ng (lane 3, 6, 9, and 13), 50 ng (lanes 4, 7, 10, and 14) and 150 ng equivalent (lanes 11 and 15) of IREF-2 proteins. Recombinant TAF-Iβ/SET protein prepared using an Escherichia. coli expression system (33, 110, and 330 ng) was also tested (lanes 16–18). After incubation at 30°C for 2 hr, the RNA products were collected and analyzed by 10% Urea-PAGE followed by autoradiography. (B) Template preference of IREF-2-dependent viral RNA synthesis. Viral RNA replication reactions were performed in the cell-free viral RNA synthesis system using mnRNP and either v53 (lanes 1–5) or c53 (lanes 6–10) as viral model templates for vRNA and complementary RNA (cRNA), respectively. ApG at a final concentration of 0.2 mM (lanes 1 and 6) or 30, 100, and 300 ng of recombinant pp32 (lanes 3–5 and 8–10) was added to the reaction. (C) Effect of IREF-2 on cRNA synthesis from vRNP. Cell-free viral RNA synthesis using 2 ng PB1-equivalent of vRNP as the enzyme source and an endogenous genomic vRNA template were carried out in the presence (lane 1) or absence (lanes 2–8) of 0.2 mM ApG. Recombinant pp32 (lanes 3–5, 30, 100, and 300 ng, respectively) was added to the reactions. As a positive control, 1.5, 5, and 15 ng of recombinant IREF-1/MCM were also used (lanes 6–8). The RNA products were collected and analyzed by 4% Urea-PAGE followed by autoradiography. One-third (33%) of the total products derived from the ApG-primed cRNA synthesis were subjected to Urea-PAGE (lane 1). (D) Effect of IREF-2 on cap-snatching viral transcription. Cell-free viral RNA synthesis reactions were performed using mnRNP as the enzyme source and the exogenous model vRNA template (v53) in the presence of 0.2 mM ApG (lane 1) or globin mRNA as the 5’-capped RNA donor (lanes 2–5). Recombinant pp32 (lanes 3–5, 30, 100, and 300 ng, respectively) was added to the reaction.
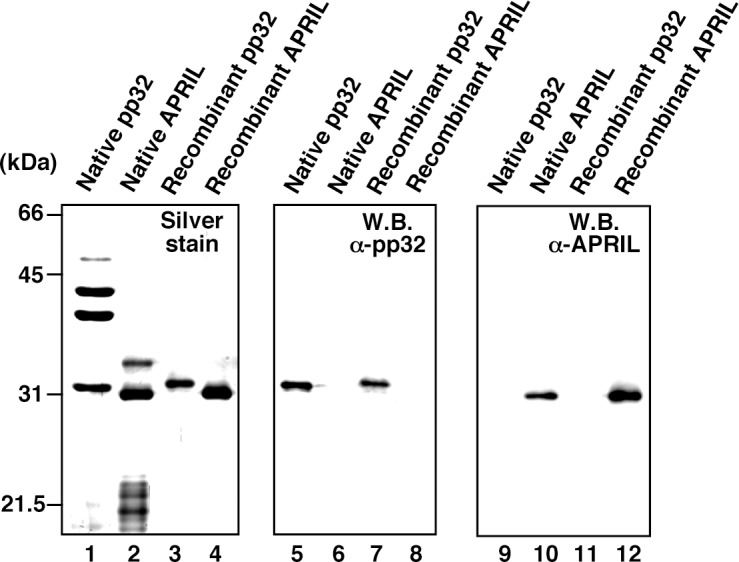
Figure 3—figure supplement 1. Protein profiles of native and recombinant influenza virus replication factor-2 (IREF-2s).