Abstract
Several previous reports suggested that many commercially available antibodies directed against G protein-coupled receptors lack sufficient selectivity. Accordingly, it has been proposed that receptor antibodies should be validated by at least one of several criteria, such as testing tissues or cells after knockout or silencing of the corresponding gene. Here, we tested whether twelve commercially available antibodies directed against α-adrenergic receptor (AR) subtypes (α1A/B/D, α2A/B/C), atypical chemokine receptor 3 (ACKR3) and vasopressin receptor 1A (AVPR1A) suffice these criteria. We detected in flow cytometry experiments with human vascular smooth muscle cells that the fluorescence signals from each of these antibodies were reduced by 46±10% – 91±2% in cells treated with commercially available siRNA specific for each receptor, as compared with cells that were incubated with non-targeting siRNA. The tested antibodies included anti-ACKR3 (R&D Systems, mab42273), for which specificity has previously been demonstrated. Staining with this antibody resulted in 72±5% reduction of the fluorescence signal after ACKR3 siRNA treatment. Furthermore, staining with anti-α1A-AR (Santa Cruz, sc1477) and anti-ACKR3 (Abcam, ab38089), which have previously been reported to be non-specific, resulted in 70±19% and 80±4% loss of the fluorescence signal after α1A-AR and ACKR3 siRNA treatment, respectively. Our findings demonstrate that the tested antibodies show reasonable selectivity for their receptor target under our experimental conditions. Furthermore, our observations suggest that the selectivity of GPCR antibodies depends on the method for which the antibody is employed, the species from which cells/tissues are obtained and on the type of specimens (cell, tissue/cell homogenate or section) tested.
Keywords: selective antibodies, alpha adrenergic receptors, vasopressin receptor, chemokine receptor
Introduction
G protein-coupled receptors (GPCR) or 7 transmembrane domain (7TM) receptors are the largest group of eukaryotic cell surface receptors and play important roles in physiology and pathology (Alexander et al., 2013, Venkatakrishnan et al., 2013, Vaidehi et al., 2014). It has been estimated that approximately 50% of all currently available drugs target GPCRs (Fredriksson and Schioth, 2005). Thus, selective antibodies that detect endogenous GPCRs can be essential for the study of these receptors (Gupta and Devi, 2006, Talmont et al., 2012). Several lines of evidence, however, suggested that many commercially available antibodies directed against GPCRs, such as histamine receptors, adrenoceptors or chemokine receptors, lack sufficient selectivity (Hamdani and van der Velden, 2009, Jensen et al., 2009, Pradidarcheep et al., 2009, Berahovich et al., 2010, Beermann et al., 2012, Bohmer et al., 2014, Cecyre et al., 2014, Cernecka et al., 2014, Talmont and Mouledous, 2014). Accordingly, it has been proposed that receptor antibodies should be validated by at least one of the following techniques: a) disappearance of staining in knock-out animals of the target receptor, b) reduction of staining upon knock-down approaches such as siRNA treatment, c) selectivity of staining in immunoblots or immunocytochemistry for the target receptor vs. related subtypes when expressed in the same cell line and/or d) antibodies raised against multiple distinct epitopes of a receptor yielding very similar staining patterns (Michel et al., 2009).
We have shown previously that several commercially available antibodies against chemokine (C-X-C motif) receptor 4 (CXCR4) show acceptable selectivity as we observed a major reduction of staining with these antibodies when endogenous CXCR4 was silenced with siRNA (Saini et al., 2010, Tripathi et al., 2015). In the present study, we again applied these criteria and tested twelve commercially available antibodies directed against GPCR targets that are in the center of our current research interests: α-adrenoceptors, atypical chemokine receptor 3 (ACKR3) and vasopressin receptor 1A (AVPR1A). The tested antibodies included two antibodies that have previously been reported to be non-specific and an antibody that has been thoroughly evaluated and found to be specific for the GPCR target (Jensen et al., 2009, Berahovich et al., 2010).
Materials and Methods
Cells and reagents
Human aortic vascular smooth muscle cells (hVSMC) were obtained from American Type Culture Collection (ATCC) and cultured as described in detail previously (Tripathi et al., 2015). Antibodies were obtained from commercial sources and are listed in Table 1. siRNA reagents were purchased from Thermo Scientific Dharmacon.
Table 1.
Antibodies tested
| Company | Catalog number | GPCR target | Ab type | Recommended applications | Recommended species | Accell siRNA (catalog number) | % reduction of signal (n) |
|---|---|---|---|---|---|---|---|
| abcam | ab137123 | α1A-AR | Rabbit mAb | Flow Cyt, ICC/IF, WB | Hu, Ms, Rt | Human ADRA1A (148) (E-005419-00-0050) | 62 ± 13 (8) |
| Santa Cruz | sc1477 | α1A-AR | Goat pAb | WB, IF, ELISA | Hu, Ms, Rt, Cow | Human ADRA1A (148) (E-005419-00-0050) | 70 ± 19 (4) |
| abcam | ab169523 | α1B-AR | Rabbit mAb | Flow Cyt, ICC/IF, WB | Hu, Ms, Rt | Human ADRA1B (147) (E-005420-00-0050) | 82 ± 19 (8) |
| abcam | ab84402 | α1D-AR | Rabbit pAb | WB, IHC-P | Hu, Ms, Rt, Dm, Zf | Human ADRA1D (146) (E-005421-00-0050) | 74 ± 22 (7) |
| Santa Cruz | sc27099 | α1D-AR | Goat pAb | WB, IF, ELISA | Hu | Human ADRA1D (146) (E-005421-00-0050) | 85 ± 3 (4) |
| Sigma | sab4500548 | α2A-AR | Rabbit pAb | IHC, WB | Hu, Ms, Rt | Human ADRA2A (150) (E-005422-00-0050) | 46 ± 10 (4) |
| abcam | ab21768 | α2B-AR | Rabbit mAb | Flow Cyt, WB, IP | Hu, Ms, Rt | Human ADRA2B (151) (E-005423-00-0050) | 60 ± 2 (4) |
| abcam | ab167433 | α2C-AR | Mouse mAb | WB, ICC | Hu, Ms, Rt, Gp | Human ADRA2C (152) (E-005424-00-0050) | 70 ± 20 (3) |
| R&D Systems | mab42273 clone 11G8 | ACKR3 | Mouse mAb | Flow Cyt, IHC | Hu | Human CXCR7 (57007) (E-013212-00-0050) | 72 ± 5 (3) |
| abcam | ab38089 | ACKR3 | Rabbit pAb | IHC-P, WB | Hu, Ms | Human CXCR7 (57007) (E-013212-00-0050) | 80 ± 4 (3) |
| LSBio | LS-B1815 | ACKR3 | Rabbit pAb | IHC-P, WB, Flow Cyt | Hu, Ms, Rt, Bt, Bo, Do, Bo, Mo, Ha, Ho, Pig, Ra | Human CXCR7 (57007) (E-013212-00-0050) | 66 ± 4 (3) |
| Bioss Antibodies | bs-11598R | AVPRA1 | Rabbit pAb | IHC-P, WB, IF | Hu, Ms, Rt | Human AVPR1A (552) (E-003631-00-0050) | 91 ± 2 (4) |
Ab: antibody. pAb: polyclonal antibody. mAb: monoclonal antibody. WB: Western blot. Flow Cyt: Flow cytometry. ICC: Immunocytochemistry. IF: Immunofluorescence. ELISA: Enzyme linked immunosorbent assay. IHC: Immunohistochemistry. IHC-P: Immunohistochemistry-paraffin protocol. Recommended species: species reactivity as provided by the antibody supplier (Hu, Human; Ms, Mouse; Rt, rat; Dm, Drosophila; Zf, Zebra fish; Gp, Guinea pig; Bt, Bat; Bo, Bovine; Do, Dog; Mo, Monkey; Ha, Hamster; Ho, Horse and Ra, Rabbit). % reduction of signal: Average percent reduction of the fluorescence signal after incubation of human vascular smooth muscle cells with receptor specific siRNA, as compared with cells after incubation with non-targeting siRNA; Data are mean ± SD. (n) = sample size – number of transfections, in parentheses.
Gene silencing by RNA interference
GPCR genes were silenced using commercially available siRNA, as described previously (Saini et al., 2010, Tripathi et al., 2015). The siRNA that we used is listed in Table 1. In brief, hVSMC were grown in 1 mL Accell siRNA delivery media per well (Thermo Scientific Dharmacon) in 12 well plates (Nunc). Accell siRNA was reconstituted with 1X siRNA buffer to a stock concentration of 100 μM. Cells were then transfected with 10 nmol siRNA and incubated for 72 h at 37°C, 5% CO2. Accell non-targeting (NT) siRNA pool was used as negative control. After 72 h, cells were assayed for receptor cell surface expression by flow cytometry. For each receptor siRNA, we performed 3–8 independent transfections. From each transfection, cells were analyzed in duplicate by flow cytometry.
Flow cytometry
Cells were labeled with primary antibodies (Table 1, all antibodies were used in a 1:200 dilution) in combination with anti-rabbit FITC conjugated goat IgG (ab6717, Abcam), anti-mouse FITC conjugated goat IgG (ab6785) or anti-goat FITC conjugated rabbit IgG (ab97104) diluted 1:70, as appropriate. Rabbit IgG (GWB-3274CD, R&D Systems) in combination with FITC-conjugated anti-rabbit goat IgG (ab6717, Abcam) was used as a negative control. The geometric fluorescence intensities of at least 3 x 104 cells were recorded and analyzed using the FlowJo software (Tree Star). All data are described as mean ± standard deviation.
Results
We compared the fluorescence signals of 12 commercially available antibodies directed against various GPCRs that were measured by flow cytometry using hVSMC after incubation with receptor specific siRNA or non-targeting siRNA. All antibodies stained the cells (relative fluorescence units (rfu) > 103–104, IgG control – rfu 155 ± 75) and responded with a reduction of the fluorescence signals after incubation of the cells with the corresponding receptor specific siRNA, when compared with cells incubated with non-targeting siRNA.
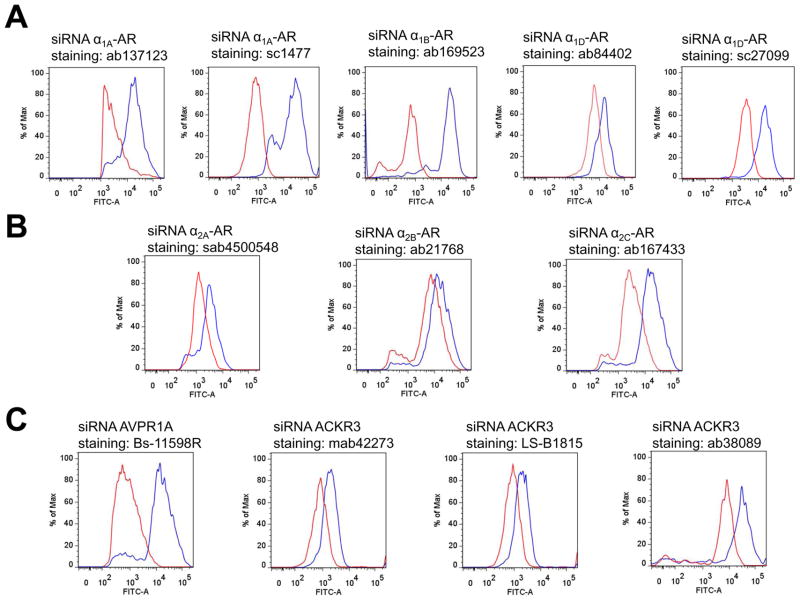
Fig. 1 shows typical analyses of the fluorescence intensities for the antibodies directed against α1-AR subtypes (Fig. 1A), α2-AR subtypes (Fig. 1B), AVPR1A and CXCR7 (Fig. 1C). For α1A-AR and α1D-AR, two different antibodies were tested. The average reduction of the fluorescence signals for α1-AR subtype antibodies ranged from 62±13% for anti-α1A-AR/ab137123 to 85±3% for anti-α1D-AR/sc27099 (Fig. 1A and Table 1). The reduction of the fluorescence signals measured with α2-AR subtype antibodies was slightly lower than with α1-AR subtype antibodies and ranged from 46±10% for anti-α2A-AR to 70±20% for anti-α2C-AR (Fig. 1B and Table 1). Fig. 1C shows typical analyses of the fluorescence intensities for an antibody directed against AVPR1A and for three different antibodies directed against ACKR3. With anti-AVPR1A, we observed 91±2% reduction of the fluorescence signal after incubation of the cells with AVPR1A siRNA. When cells were incubated with ACKR3 siRNA, the fluorescence signals were reduced by 66±4% when cells were labeled with LS-B1815, and by 72±5% and 80±4% when cells were stained with mab42273 and ab38089, respectively, as compared with cells incubated with non-targeting siRNA (Table 1).
Figure 1.
Assessment of α1- (A.), α2 (B.) -adrenoceptor subtype antibodies and AVPR1A and ACKR3 antibodies (C.) by flow cytometry. Human VSMC were transfected with receptor-specific siRNA (red lines) or non-targeting siRNA (blue lines). The siRNA that was used for transfection and the antibodies employed for flow cytometry are provided. A. from left to right: anti-α1A-AR/ab137123, anti-α1A-AR/sc1477, anti-α1B-AR/ab169523, anti-α1D-AR/ab84402 and anti-α1D-AR/sc27099. B. from left to right: anti-α2A-AR/sab4500548, anti-α2B-AR/ab21768 and anti-α2C-AR/ab167433. C. from left to right: anti-AVPR1A/bs11598R, anti-ACKR3/mab42273, anti-ACKR3/LS-B1815 and anti-ACKR3/ab38089.
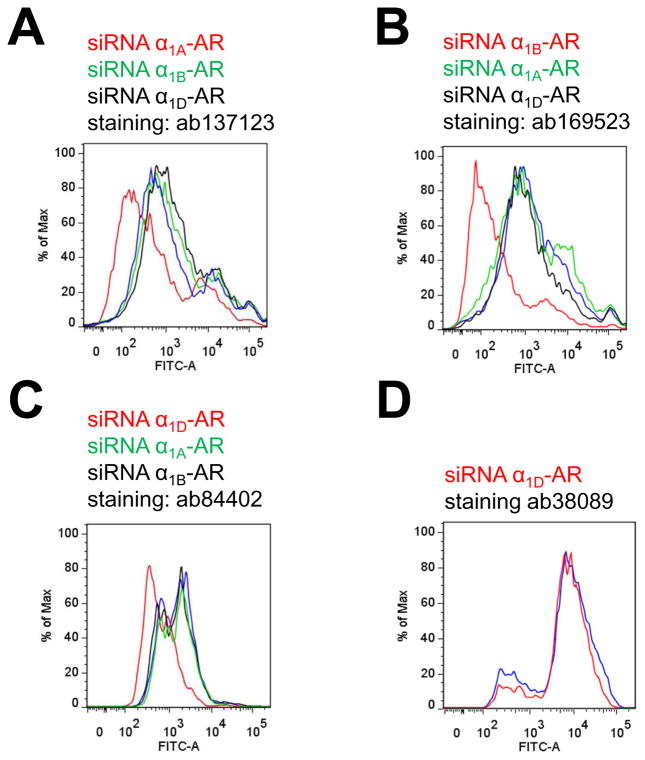
As another selectivity control for the α1-AR subtype antibodies, we then incubated cells with non-targeting siRNA or siRNA for each α1-AR subtype and tested anti-α1A-AR/ab137123, anti-α1B-AR/ab169523 and anti-α1D-AR/ab84402 for each condition by flow cytometry (Fig. 2A–C). As shown in Table 2, when cells were incubated with receptor specific siRNA corresponding to the GPCR target of each antibody, the fluorescence signals were reduced by 68±10% after staining with anti-α1A-AR/ab137123, and by 91±3% and 97±1% after staining with anti-α1B-AR/ab169523 (Fig. 2B) and anti-α1D-AR/ab84402 (Fig. 2C), respectively, as compared with cells incubated with non-targeting siRNA. The fluorescence intensities measured after incubation of cells with siRNA targeting other α1-AR subtypes, however, were comparable to the fluorescence signals from cells incubated with non-targeting siRNA for each antibody (Fig. 2A–C and Table 2). Furthermore, when cells were stained with anti-ACKR3/ab38089, the fluorescence signals were indistinguishable in cells incubated with α1D-AR siRNA or non-targeting siRNA (Fig. 2D).
Figure 2.
Selectivity testing of α1-AR subtype antibodies. Human VSMC were transfected with receptor-specific siRNA or non-targeting siRNA (blue lines). The siRNA that was used for transfection (same color code as lines) and the antibodies employed for flow cytometry are provided. A. Cells transfected with siRNA targeting α1A-AR (red), α1B-AR (green) and α1D-AR (black) and stained with anti-α1A-AR/ab137123. B. Cells transfected with siRNA targeting α1A-AR (green), α1B-AR (red) and α1D-AR (black) and stained with anti-α1B-AR/ab169523. C. Cells transfected with siRNA targeting α1A-AR (green), α1B-AR (black) and α1D-AR (red) and stained with anti-α1D-AR/ab38089. D. Cells transfected with siRNA targeting α1D-AR (red) and stained with anti-ACKR3/ab38089.
Table 2.
Selectivity test of α1-AR subtype antibodies
| Antibody | GPCR target | %RFU | ||
|---|---|---|---|---|
| cells incubated with siRNA targeting | ||||
| α1A-AR | α1B-AR | α1D-AR | ||
|
| ||||
| ab137123 | α1A-AR | 32 ± 10 | 95 ± 3 | 127 ± 28 |
|
| ||||
| ab169523 | α1B-AR | 85 ± 16 | 9 ± 3 | 95 ± 15 |
|
| ||||
| ab84402 | α1D-AR | 103 ± 3 | 99 ± 6 | 3 ± 1 |
Cells (human vascular smooth muscle cells) were incubated with non-targeting siRNA or siRNA targeting α1-AR subtypes and then analyzed by flow cytometry utilizing the antibodies listed. %RFU: Relative fluorescence units in percent of the fluorescence units measured after incubation of cells with non-targeting siRNA. Data are mean ± standard deviation, n = 3.
Discussion
In the present study, we utilized hVSMC to evaluate selectivity of 12 commercially available antibodies directed against GPCR targets, which are known to be expressed in these cells (Alexander et al., 2013). Our results demonstrate that the tested GPCR antibodies reported with a major reduction of staining when hVSMC were transfected with the corresponding receptor-specific siRNA. According to criteria proposed by Michel et al., this constitutes reasonable evidence for the specificity of the tested antibodies (Michel et al., 2009). Previously, we have utilized the various α-AR subtype antibodies in proximity ligation assays with hVSMC and detected that these antibodies resulted in distinct signal patterns (Tripathi et al., 2015). The finding of the present study that α1-AR subtype antibodies reported with a significant reduction of the fluorescence signals from flow cytometry experiments only when the corresponding receptor subtype gene was silenced with siRNA is in agreement with our previous observations and further indicates selectivity of the antibodies for their receptor targets (Michel et al., 2009, Tripathi et al., 2015).
Every antibody likely shows some degree of non-specific binding and siRNA gene silencing is not expected to fully abrogate expression of the target gene. Thus, the degree of reduction in staining that is required to confidently assume antibody selectivity could be a matter of debate. Anti-ACKR3 (mab42278, clone 11G8) has previously been validated for immunohistochemistry and flow cytometry utilizing the current gold standard, the use of tissues/cells in which the target gene is missing (Berahovich et al., 2010). We observed a 72% reduction of the flow cytometry signal after transfection of cells with ACKR3 siRNA when this antibody was employed. When a reduction in signal by 70% or more is considered as a benchmark for excellent target selectivity in our assay, 8 of the tested antibodies would be considered to show excellent selectivity. Nevertheless, the remaining 4 antibodies would still show at least good or sufficient selectivity to report alterations in the expression levels of their endogenous protein targets.
Interestingly, anti-α1A/sc1477 and anti-ACKR3/ab38089 have previously been reported to be non-specific, as assessed by Western blot experiments or immunohistochemistry with tissues from knockout mice (Jensen et al., 2009, Berahovich et al., 2010). Under our experimental conditions, however, both antibodies showed excellent selectivity, comparable or better than anti-ACKR3/mab42278 (clone 11G8).
In combination with previous observations that a β3-AR antibody was non-selective in Western blot experiments but selective when used for immunohistochemistry (Cernecka et al., 2014), these data suggest that antibodies can display different degrees of selectivity in different assays, different species or tissues and when different types of specimens, such cells, homogenates or tissue sections, are being evaluated. This phenomenon is likely related to the fact that, depending on the application and experimental conditions, the probed epitopes can be linear or three-dimensional, completely exposed and completely or partially masked by post-translational modifications. Vice versa, it may also be possible that an antibody that has been validated in tissues that lack the target gene does not show selectivity when tested in another tissue, species or application.
In conclusion, we have validated 12 commercially available antibodies directed against GPCRs when employed for flow cytometry with hVSMC. The majority of these antibodies showed excellent selectivity for the endogenous GPCR target under our experimental conditions. Such antibody validation, however, is labor intensive and time consuming. Furthermore, the costs for siRNA reagents are high and may not be affordable for every research laboratory. As the antibodies are being sold for profit, we believe that commercial antibody provider should share this burden and validate an antibody for the application and species for which it is marketed. This will likely reduce the risk for the individual investigator to waste the limited financial resources that are currently available to the majority of the scientific community. We strongly concur with the criteria proposed by Michel et al. and recommend confirmation of antibody selectivity under the specific experimental conditions for which the antibodies are being employed. While this will improve scientific rigor, it will also increase costs for labor and supplies. We share the opinion that requests for additional funding for antibody validation should be proposed in research funding applications and be honored by the peer-reviewers and funding agencies.
Acknowledgments
This research was supported by National Institute of General Medical Sciences (Awards R01GM107495 and T32GM008750); by the National Cancer Institute (Awards R01CA135341 and R01CA188427); by the National Heart, Lung, and Blood Institute (Award R21HL118588); and by grant that was awarded and administered by the U.S. Army Medical Research and Materiel Command (USAMRMC)/ Medical Research Acquisition Activity (USAMRAA) at Fort Detrick, MD, under Contract Number W81XWH-15-1-0262.
Footnotes
The content is solely the responsibility of the authors.
References
- Alexander SP, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, Collaborators C. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beermann S, Seifert R, Neumann D. Commercially available antibodies against human and murine histamine H(4)-receptor lack specificity. Naunyn Schmiedebergs Arch Pharmacol. 2012;385:125–135. doi: 10.1007/s00210-011-0700-4. [DOI] [PubMed] [Google Scholar]
- Berahovich RD, Penfold ME, Schall TJ. Nonspecific CXCR7 antibodies. Immunology letters. 2010;133:112–114. doi: 10.1016/j.imlet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- Bohmer T, Pfeiffer N, Gericke A. Three commercial antibodies against alpha1-adrenergic receptor subtypes lack specificity in paraffin-embedded sections of murine tissues. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:703–706. doi: 10.1007/s00210-014-0992-2. [DOI] [PubMed] [Google Scholar]
- Cecyre B, Thomas S, Ptito M, Casanova C, Bouchard JF. Evaluation of the specificity of antibodies raised against cannabinoid receptor type 2 in the mouse retina. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:175–184. doi: 10.1007/s00210-013-0930-8. [DOI] [PubMed] [Google Scholar]
- Cernecka H, Pradidarcheep W, Lamers WH, Schmidt M, Michel MC. Rat beta(3)-adrenoceptor protein expression: antibody validation and distribution in rat gastrointestinal and urogenital tissues. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:1117–1127. doi: 10.1007/s00210-014-1039-4. [DOI] [PubMed] [Google Scholar]
- Fredriksson R, Schioth HB. The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol. 2005;67:1414–1425. doi: 10.1124/mol.104.009001. [DOI] [PubMed] [Google Scholar]
- Gupta A, Devi LA. The use of receptor-specific antibodies to study G-protein-coupled receptors. Mt Sinai J Med. 2006;73:673–681. [PubMed] [Google Scholar]
- Hamdani N, van der Velden J. Lack of specificity of antibodies directed against human beta-adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:403–407. doi: 10.1007/s00210-009-0392-1. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Wieland T, Tsujimoto G. How reliable are G-protein-coupled receptor antibodies? Naunyn Schmiedebergs Arch Pharmacol. 2009;379:385–388. doi: 10.1007/s00210-009-0395-y. [DOI] [PubMed] [Google Scholar]
- Pradidarcheep W, Stallen J, Labruyere WT, Dabhoiwala NF, Michel MC, Lamers WH. Lack of specificity of commercially available antisera against muscarinergic and adrenergic receptors. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:397–402. doi: 10.1007/s00210-009-0393-0. [DOI] [PubMed] [Google Scholar]
- Saini V, Marchese A, Majetschak M. CXC chemokine receptor 4 is a cell surface receptor for extracellular ubiquitin. J Biol Chem. 2010;285:15566–15576. doi: 10.1074/jbc.M110.103408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmont F, Mouledous L. Evaluation of commercial antibodies against human sphingosine-1-phosphate receptor 1. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:427–431. doi: 10.1007/s00210-014-0957-5. [DOI] [PubMed] [Google Scholar]
- Talmont F, Mouledous L, Boue J, Mollereau C, Dietrich G. Denatured G-protein coupled receptors as immunogens to generate highly specific antibodies. PLoS One. 2012;7:e46348. doi: 10.1371/journal.pone.0046348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi A, Vana PG, Chavan TS, Brueggemann LI, Byron KL, Tarasova NI, Volkman BF, Gaponenko V, Majetschak M. Heteromerization of chemokine (C-X-C motif) receptor 4 with alpha1A/B-adrenergic receptors controls alpha1-adrenergic receptor function. Proc Natl Acad Sci U S A. 2015;112:E1659–1668. doi: 10.1073/pnas.1417564112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidehi N, Bhattacharya S, Larsen AB. Structure and dynamics of G-protein coupled receptors. Adv Exp Med Biol. 2014;796:37–54. doi: 10.1007/978-94-007-7423-0_3. [DOI] [PubMed] [Google Scholar]
- Venkatakrishnan AJ, Deupi X, Lebon G, Tate CG, Schertler GF, Babu MM. Molecular signatures of G-protein-coupled receptors. Nature. 2013;494:185–194. doi: 10.1038/nature11896. [DOI] [PubMed] [Google Scholar]