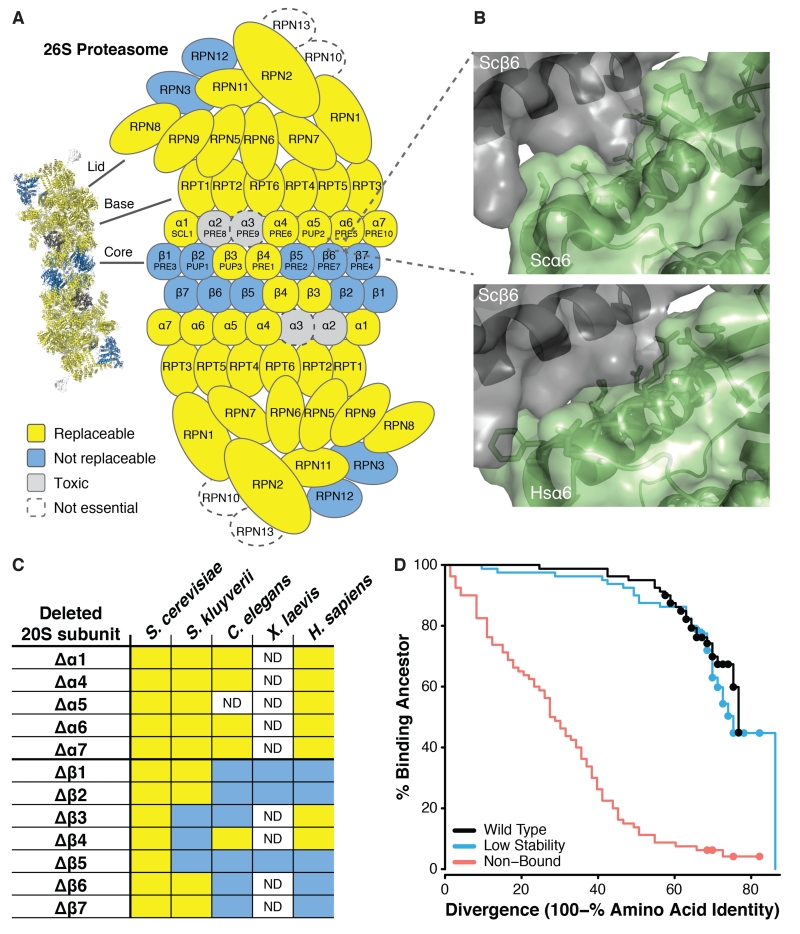
Fig. 4. Proteasome subunits are differentially replaceable.
(A) Yeast 26S proteasome genes were generally replaceable, except for two interacting clusters, in the 19S regulatory “lid” particle and in the 20S core β-subunit ring. (B) The yeast α6-β6 subunit interface (top panel) sterically accommodates the human subunit (bottom panel, showing superposition of human α6 onto the yeast α6) despite 50% sequence identity at the interface. (C) Alpha subunits from diverse eukaryotes generally complemented the yeast mutant, but not beta subunits (unlike plasmid-expressed S. cerevisiae genes, included as positive controls). (D) In simulated evolution of interacting proteins Ubc9 and Smt3, if binding to the extant partner is not enforced (“Non-Bound”) a protein’s ability to bind its ancestral partner decays rapidly as sequences diverge. However, if extant binding is enforced (“Wild Type” and “Low Stability”), even highly diverged proteins often still bind to their ancestral partners. (Dots indicate right-censored data; see Fig. S14.)