Figure 4.
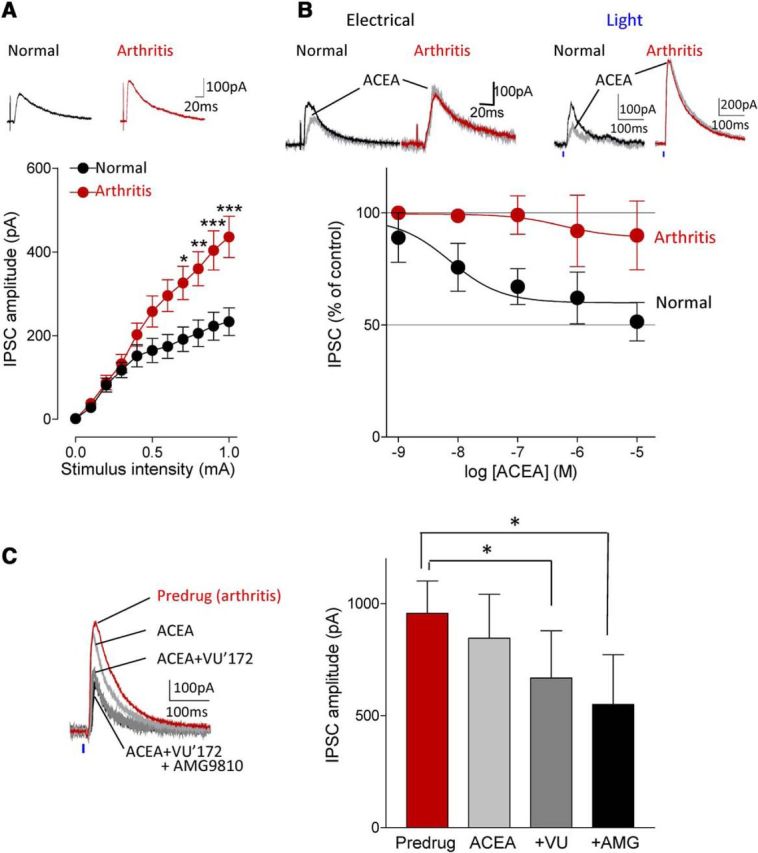
Coactivation of CB1 and mGluR5 inhibits enhanced inhibitory synaptic transmission in the arthritis pain model. A, Input-output functions of IPSCs recorded in slices from arthritic rats (n = 34 neurons) were significantly different (F(1,605) = 40.84, p < 0.0001, main effect of arthritis, two-way ANOVA) from normal controls (n = 23 neurons). IPCSs could be blocked with bicuculline or NBQX (data not shown). *,**,***p < 0.05–0.001, compared with normal (Bonferroni posttests). B, ACEA (10 nm) decreased IPSCs evoked by electrical or optical stimulation under normal condition but had no significant effect in the arthritis pain model. Concentration-response curves for ACEA under normal conditions (n = 4–8 neurons) and in the arthritis pain model (n = 3–8 neurons) were significantly different (p < 0.001; F(1,39) = 16.33, two-way ANOVA). C, Application of ACEA (10 nm) alone had no effect but addition of VU'172 (1 μm) decreased IPSCs in the pain model. The inhibitory effect of the combination persisted in the presence of a TRPV1 receptor antagonist AMG9810 (10 μm, n = 5 neurons; same neurons were tested with ACEA alone, ACEA and VU'172, and addition of AMG9810). *p < 0.05, F(1,12) = 56.23, repeated-measures ANOVA with Bonferroni posttests compared with predrug. A–C, Current traces show IPSCs (average of 8–10) evoked with electrical stimulation of 0.6 and 0.8 mA (A, B) and with optical activation of BLA terminals with 40 mW (at laser source; power density, 2.17 mW/mm2).
