Abstract
Lack or downregulation of the dopamine D2 receptor (D2R) results in increased renal expression of injury markers and proinflammatory factors that is independent of a blood pressure increase. This study aimed to determine the mechanisms involved in the regulation of renal inflammation by D2Rs. Silencing D2Rs in mouse renal proximal tubule cells increased the expression of the proinflammatory TNF-α, monocyte chemoattractant protein-1 (MCP-1), and IL-6. D2R downregulation also increased Akt phosphorylation and activity, and glycogen synthase kinase-3β (GSK3β) phosphorylation and cyclin D1 expression, downstream targets of Akt; however. phosphatidylinositol 3-kinase (PI3K) activity was not affected. Conversely, D2R stimulation decreased Akt and GSK3β phosphorylation and cyclin D1 expression. Increased phospho-Akt, in the absence of increased PI3K activity, may result from decreased Akt dephosphorylation. Inhibition of protein phosphatase 2A (PP2A) with okadaic acid reproduced the effects of D2R downregulation on Akt, GSK3β, and cyclin D1. The PP2A catalytic subunit and regulatory subunit PPP2R2C coimmunoprecipitated with the D2R. Basal phosphatase activity and the expression of PPP2R2C were decreased by D2R silencing that also blunted the increase in phosphatase activity induced by D2R stimulation. Similarly, silencing PPP2R2C also increased the phosphorylation of Akt and GSK3β. Moreover, downregulation of PPP2R2C resulted in increased expression of TNF-α, MCP-1, and IL-6, indicating that decreased phosphatase activity may be responsible for the D2R effect on inflammatory factors. Indeed, the increase in NF-κB reporter activity induced by D2R silencing was blunted by increasing PP2A activity with protamine. Our results show that D2R controls renal inflammation, at least in part, by modulation of the Akt pathway through effects on PP2A activity/expression.
Keywords: dopamine D2 receptor, renal proximal tubule cells, protein phosphatase 1A, AKT
independent of innervation, the kidney synthesizes dopamine that is important in the regulation of renal function and blood pressure (1). The renal dopaminergic system also regulates the production of reactive oxygen species and the inflammatory reaction (2, 3, 11, 52, 53, 56), both involved in the development of renal injury and ultimately in the induction and maintenance of hypertension (17). Mice with intrarenal dopamine deficiency have increased oxidative stress, as well as increased inflammatory and tubular injury markers in the kidney (55), and decreased renal dopamine production aggravates angiotensin II-mediated renal injury (52).
We have shown that the dopamine D2 receptor (D2R) in the kidney has a direct and significant role in regulating the mechanisms involved in the development of renal inflammation and injury, as well as in blood pressure control (2, 28). Mice with a lack or reduced level of D2R expression and function in the kidney have increased renal expression of proinflammatory and decreased expression of anti-inflammatory cytokines/chemokines, as well as histological and functional evidence of renal inflammation and injury. These findings suggest that the D2R has protective effects in the kidney by limiting the inflammatory reaction and that impaired function of the D2R results in renal inflammation and organ damage (56).
Renal proximal tubule cells (RPTCs) produce both pro- and anti-inflammatory cytokines and chemokines (40), which are secreted across their apical and basolateral membranes (49) and contribute to the development and progression of glomerular and tubular injury. However, the factors that regulate cytokine production in these cells are incompletely understood. We have shown that the D2R is one of the factors that regulate cytokine production in mouse RPTCs. Silencing the D2R in RPTCs in culture increased the expression of proinflammatory factors while D2R stimulation reduced the inflammatory effects of angiotensin II (56). Moreover human RPTCs bearing single nucleotide polymorphisms of the D2R that result in decreased expression of the receptor have increased expression of inflammatory factors and a profibrotic phenotype (23).
The present study was aimed at determining the mechanisms involved in the D2R regulation of cytokine production in mouse RPTCs. Our results show that the protective effects of the D2R on renal inflammation are, at least in part, mediated by modulation of the Akt pathway, through effects on protein phosphatase 2A (PP2A) activity and expression.
MATERIALS AND METHODS
Cell culture.
Undifferentiated mouse renal cells were cultured from progenitor kidney cells, kindly supplied by Dr. Ulrich Hopfer (School of Medicine, Case Western Reserve University), isolated from mouse embryo kidneys and maintained following the procedure described by Woost et al. (50). The cells that differentiated to mouse RPTCs were maintained in DMEM/F-12 (Invitrogen) with 10% fetal bovine serum, 100 IU/ml penicillin, 100 IU/ml streptomycin, and 250 μg/ml amphotericin B. Cells with low passage numbers (<18) were used to avoid the confounding effects of cellular senescence. Mouse RPTCs were cultured to 60–70% confluence and transfected (Hyperfect, Qiagen, Valencia, CA) with non-silencing small interfering (si) RNA (30 nmol/l; All stars, Qiagen), Drd2 siRNA (30 nmol/l, Qiagen), or PP2A catalytic subunit and regulatory subunit (PPP2R2C) siRNA (30 nmol/l, Qiagen). The cells were harvested after 72 h of treatment. The cell lines tested negative for Mycoplasma infection.
D2R-deficient mice.
The original F2 hybrid strain (129/SvXC57BL/6J, Oregon Health Sciences University) that contained the mutated Drd2 allele (D2−/−) was bred onto the C57BL/6J background for >20 generations (28). All animal-related studies were approved by the Institutional Animal Care and Use Committee. D2−/− mice and their wild-type littermates (D2+/+) were studied at 6–8 mo of age.
Coimmunoprecipitation.
Coimmunoprecipitation was performed using an Immunoprecipitation kit (protein G, Roche Applied Science, Indianapolis, IN). The mouse RPTC lysates were prepared using lysis buffer (50 mm Tris·HCl, pH 7.5, 150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate) with proteinase inhibitors. Equal amounts of cell lysates (500 μg protein) were mixed with normal rabbit IgG (Santa Cruz Biotechnology, Santa Cruz, CA) as a negative control, monoclonal anti-D2R (Abnova, Walnut, CA), monoclonal anti-PPP2R2C (Novus Biologicals, Littleton, CO), or monoclonal PP2A catalytic subunit (Millipore, Billerica, MA). Protein G-agarose beads (Roche) were added and incubated overnight. The immune complexes were pelleted out, and the bound proteins were eluted using 30 μl of Laemmli buffer. The samples were subjected to immunoblotting using a polyclonal anti-PPP2R2C antibody (Novus Biologicals), polyclonal anti-PP2A catalytic subunit antibody (Upstate Biotechnology, Lake Placid, NY), or polyclonal anti-D2R (Millipore).
Immunoblotting.
Mouse RPTC lysates were subjected to immunoblotting as described previously (2, 23, 56). The primary antibodies used were rabbit polyclonal p-Akt (Thr 308, Abcam, Cambridge, MA); rabbit polyclonal anti-Akt (Abcam); rabbit polyclonal anti-D2R (Millipore); monoclonal anti-glycogen synthase kinase-3β (GSK3β; Cell Signaling Technology, Danvers, MA); rabbit polyclonal anti-p-GSK3β (Cell Signaling Technology); monoclonal anti-cyclin D1 (Cell Signaling Technology); rabbit polyclonal anti-TNF-α (Abcam); rabbit polyclonal anti-monocyte chemoattractant protein-1 (MCP-1; Millipore); rabbit polyclonal anti-IL-6 (Abcam); polyclonal anti-GAPDH (Sigma-Aldrich, St. Louis, MO); monoclonal anti-PPP2R2C (Novus Biologicals); and polyclonal anti-PP2A catalytic subunit (Millipore). The densitometry values were corrected by the expression of GAPDH and are shown as the percentage of the mean density of the control group.
Quantitative real-time PCR.
Total RNA was purified using the RNeasy RNA Extraction Mini kit (Qiagen, Valencia, CA). RNA samples were converted into first-strand cDNA using an RT2 First Strand kit, following the manufacturer's protocol (Qiagen). Quantitative gene expression was analyzed by real-time PCR, performed on an ABI Prism 7900 HT (Applied Biosystems, Foster City, CA). The assay used gene-specific primers (Qiagen) and the SYBR Green real-time PCR detection method (Qiagen) and was performed as described in the manufacturer's manual. Primers used were as follows: TNF-α: PPM03113F; MCP-1: PPM03151F; and IL-6 and GAPDH: PPM02946E. Data were analyzed using the ΔΔCt method (29).
Whole cell phosphorylation assay.
Phosphatase activity was measured using the SensoLyte FDP Protein Phosphatase Assay Kit (AnaSpec, Freemont, CA), which is optimized to detect protein phosphatase activity using 3,6-fluorescein diphosphate (FDP) as a fluorogenic phosphatase substrate, following the manufacturer's procedures. Mouse RPTCs were treated with vehicle or okadaic acid (2 nM) for 60 min. Another set of mouse RPTCs was transfected with mouse D2R siRNA or non-silencing siRNA and grown to 90% confluence and then treated with the D2R agonist quinpirole (1 μM) at three time points (0, 30, and 60 min). Thereafter, the cells were lysed and protein phosphatase-containing lysates were placed in 50 μl/well and mixed the same volume of FDP reaction solution by shaking gently for 30 s. The signal of fluorescein as the final hydrolytic product of FDP was read by a Victor3 multilabel reader at Ex/Em = 485 ± 20/528 ± 20 nm. All assays were performed in duplicate.
Akt kinase assay.
The Akt kinase activity was measured using an AKT Kinase Assay Kit (Cell Signaling Technology). The D2R- transfected cells were washed twice in cold phosphate-buffered saline and then lysed on ice. The extracts were centrifuged to remove the cellular debris. Equal amounts of lysates (300 μg protein) were incubated with gentle rocking at 4°C overnight with immobilized anti-Akt antibody cross-linked to agarose hydrazide beads. After Akt was selectively immunoprecipitated from the cell lysates, the immunoprecipitated products were washed and the samples were resuspended in 50 μl of kinase assay buffer containing 200 μm ATP and 1 μg of GSK3α fusion protein, according to the manufacturer's instructions. The kinase reaction was allowed to proceed at 30°C for 30 min and stopped by the addition of Laemmli SDS sample buffer. The reaction products were resolved by 10% SDS-PAGE followed by Western blotting with an anti-phospho-GSK3α/β antibody and anti-horseradish peroxidase-conjugated anti-rabbit antibody. All assays were performed in duplicate.
Phosphatidylinositol 3-kinase assay.
Mouse RPTCs grown at 60–80% confluence were washed in ice-cold buffer and lysed in 200 μl ice-cold lysis buffer. Phosphatidylinositol 3-kinase (PI3K) was immunoprecipitated with 5 μl of rabbit antibody against full-length PI3K (Millipore), which precipitates the p110 catalytic subunit of PI3K, and 60 μl of protein A-Sepharose beads (Roche Applied Science). PI3K activity in the immunoprecipitates was measured with a PI3K ELISA (Echelon Biosciences, Salt Lake City, UT), according to the manufacturer's instructions. Briefly, immunoprecipitated enzyme and PI(4,5)P2 substrate were incubated for 1 h at room temperature in the reaction buffer. The kinase reaction was stopped by pelleting the beads by centrifugation and incubation overnight at 4°C with a PI(3,4,5)P3 detector protein, then added to the PI(3,4,5)P3-coated microplate for 1 h for competitive binding. A peroxidase-linked secondary detection reagent and colorimetric detection (absorbance was measured at 450 nm) was used to detect PI(3,4,5)P3 detector protein binding to the plate. The colorimetric signal is inversely proportional to the amount PI(3,4,5)P3 produced by PI3K.
Reporter assay.
NF-κB activation was analyzed via the transient expression of an NF-κB luciferase reporter system (Cignal Reporter Assay, Qiagen). Cells were treated with D2R-specific siRNA or non-silencing siRNA, as described above. After 48 h, the cells were treated with the PP2A activator protamine (5 μM) or vehicle and seeded for transfection with the NF-κB luciferase reporter construct. The assay was performed following the manufacturer's procedures.
Statistical analysis.
The data are expressed as means ± SE. Comparisons between two groups used the Student's t-test. One-way ANOVA followed by the Newman-Keuls test was used to assess significant differences in more than two groups. P < 0.05 was considered significant.
RESULTS
D2R regulates the expression of proinflammatory factors and Akt activity in mouse proximal tubule cells.
In mouse RPTCs, treatment with D2R siRNA significantly decreased D2R expression compared with cells treated with non-silencing siRNA (Fig. 1). Downregulation of the D2R increased the mRNA expression of TNF-α, MCP-1, and IL-6 in these cells (Fig. 1), as we have reported previously in mouse renal cortex (56).
Fig. 1.
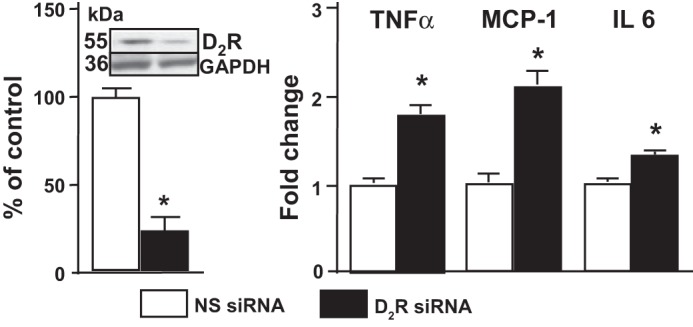
The dopamine D2 receptor (D2R) inhibits expression of proinflammatory factors. Expression of D2R determined by Western blotting and expression of TNF-α, monocyte chemoattractant protein-1 (MCP-1), and IL-6 mRNA in mouse renal proximal tubule cells (RPTCs) transfected with non-silencing small interference RNA (NS siRNA) or D2R siRNA is shown. After 48 h, the cells were washed and lysed. mRNA expression was determined by qRT-PCR. Results were corrected for expression of GAPDH mRNA and expressed as fold-change compared with the expression in NS-transfected cells. *P < 0.05 vs. NS; n = 4/group.
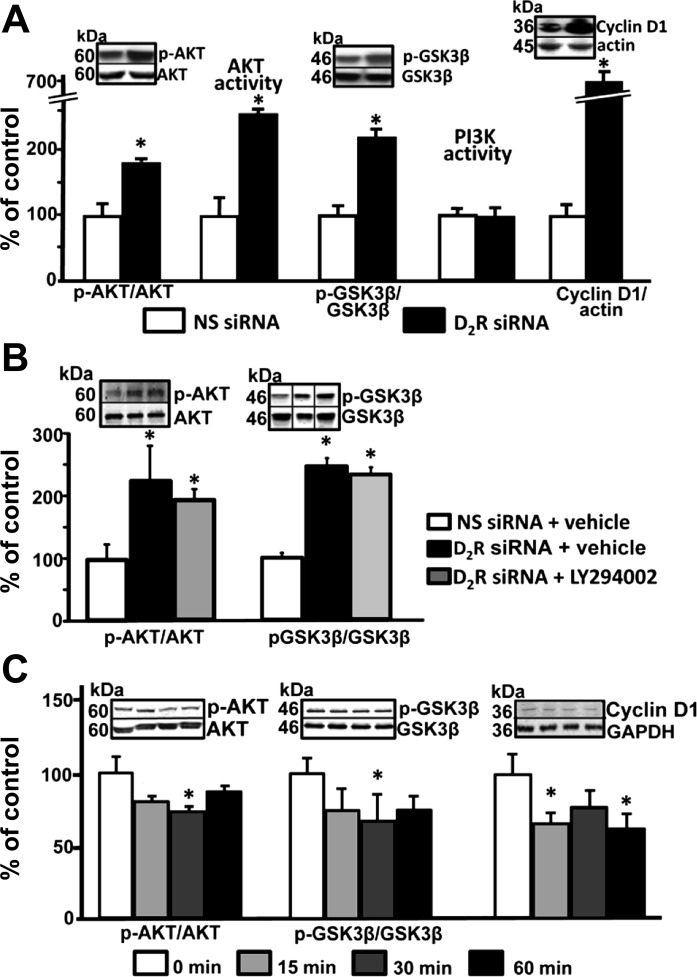
The PI3K-Akt pathway is one of the main pathways involved in the inflammatory response (10). We found that Akt phosphorylation and activity were higher in mouse RPTCs transfected with D2R siRNA than in cells transfected with non-silencing siRNA. The ratio of phosphorylated Akt to total Akt was also increased in the renal cortex of D2−/− mice compared with D2+/+ littermates (245 ± 30 vs .100 ± 10%; P < 0.03). Phosphorylation of GSK3β and expression of cyclin D1, downstream targets of Akt, were also increased in mouse RPTCs, indicating activation of the Akt pathway. However, this finding did not correlate with PI3K activity in mouse RPTCs, in which the D2R was silenced: PI3K activity was not different between RPTCs with silenced D2R and those with no D2R downregulation (Fig. 2A). To confirm that the effects on Akt are not dependent on PI3K activity, mouse RPTCs with silenced D2Rs were treated with the PI3K inhibitor LY294002. LY 294002 had no significant effect on the increased phosphorylation of Akt and GSK3β (Fig. 2B), indicating that the D2R regulates Akt function independently of PI3K activity.
Fig. 2.
The D2R regulates Akt activity. A: mouse RPTCs were transfected with NS siRNA or D2R siRNA. After 72 h, the cells were washed and lysed. Protein expression of Akt, phosphorylated Akt (p-Akt), glycogen synthase kinase-3β (GSK3β), phosphorylated GSK3β (p- GSK3β), and cyclin D1 were quantified by immunoblotting. Akt and PI3K activities were determined by enzymatic assays. Inset: 1 set of immunoblots. Results of p-Akt/Akt, Akt activity, p-GSK3 β/GSK3β, phosphatidylinositol 3-kinase (PI3K) activity, and cyclin D1/actin are expressed as percentage of NS siRNA. *P < 0.05 vs. NS siRNA; n = 4/group. B: mouse RPTCs were transfected with NS siRNA or D2R siRNA for 48 h after which they were treated for 24 h with vehicle or LY294002 (1 μM). Protein expression of Akt, p-Akt, GSK3β, and p-GSK3β were quantified by immunoblotting. Results of p-Akt/Akt and p-GSK3 β/GSK3β are expressed as percentage of NS siRNA. Black vertical lines in the blots denote rearrangement to better reflect the results shown in the bar graphs. *P < 0.05 vs. NS siRNA; n = 3–4/group. C: mouse RPTCs were treated with the D2R agonist quinpirole for the indicated time periods. Protein expression of Akt, p-Akt, GSK3β, p-GSK3β, and cyclin D1 were determined as above. Results of p-Akt/Akt, Akt activity, p-GSK3 β/GSK3β, PI3K activity, and cyclin D1/actin are expressed as percentage of time 0. *P < 0.05 vs. time 0; n = 4/group.
In contrast, treatment of mouse RPTCs with quinpirole, a D2R agonist, decreased Akt phosphorylation as well as activity as shown by decreased GSk3β phosphorylation and expression of cyclin D1 (Fig. 2C).
D2R regulates protein phosphatase activity.
Akt activity is the result of the balance between its phosphorylation-activation by PI3K-mediated signaling and its dephosphorylation by protein phosphatases (19). Protein phosphatase 2A (PP2A) is a serine/threonine phosphatase that has been shown to negatively regulate Akt activity in several tissues (4, 30, 47) and is expressed in renal proximal tubules (54). Inhibition of PP2A activity with okadaic acid, at a concentration that does not affect other phosphatases (2 nM) (12), decreased total protein phosphatase activity by 28% and increased phosphorylated Akt and GSK3β, indicating that PP2A is involved in Akt dephosphorylation in mouse RPTCs (Fig. 3A). These results demonstrate that PP2A is involved in the regulation of the Akt-GSK3β pathway. To determine whether D2R function affects PP2A activity, we treated mouse RPTCs with a D2R agonist. The stimulation of D2Rs resulted in a significant (22%) increase in total phosphatase activity at 15 min of agonist treatment (Fig. 3B). In contrast, silencing the D2R in these cells decreased basal phosphatase activity by 20% and abolished the increase in activity induced by the D2R agonist (Fig. 3B). These results indicate that D2R function is associated with an increase in PP2A phosphatase activity.
Fig. 3.
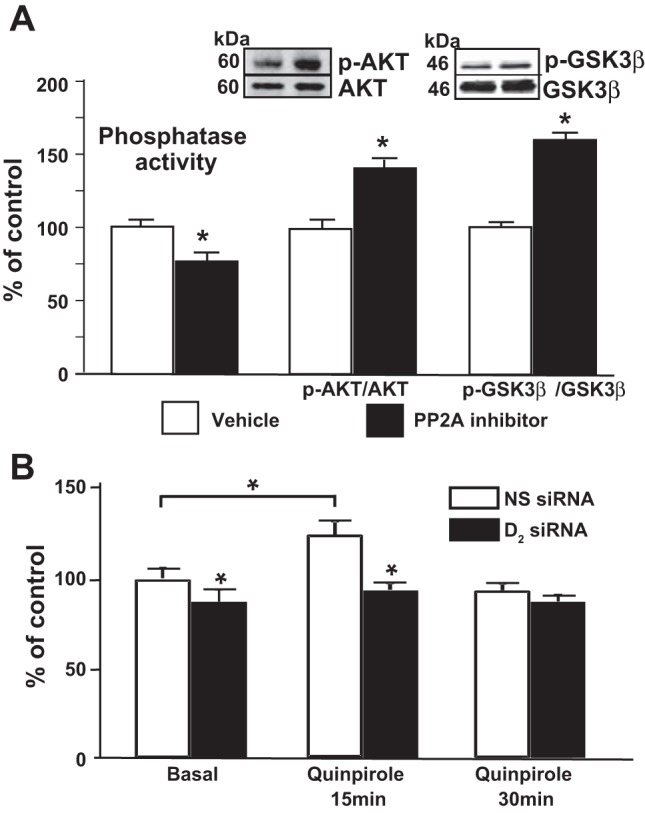
The D2R regulates phosphatase activity. A: mouse RPTCs were treated with vehicle or with the PP2A inhibitor okadaic acid (2 nM). Phosphatase activity was assayed using a fluorogenic substrate as described in materials and methods. Protein expression of Akt, p-Akt, GSK3β, and p-GSK3β was quantified by immunoblotting. Inset: 1 set of immunnoblots. Results are expressed as percentage of vehicle. *P < 0.05 vs. vehicle; n = 5/group. B: mouse RPTCs transfected with NS siRNA or D2R siRNA were treated with quinpirole (1 μM) or vehicle for the indicated time periods. Phosphatase activity was determined as above. Results are expressed as percentage of NS siRNA at time 0. *P < 0.05 vs. basal 0; n = 4/group.
D2R regulates PPP2R2C, a regulatory subunit of PP2A.
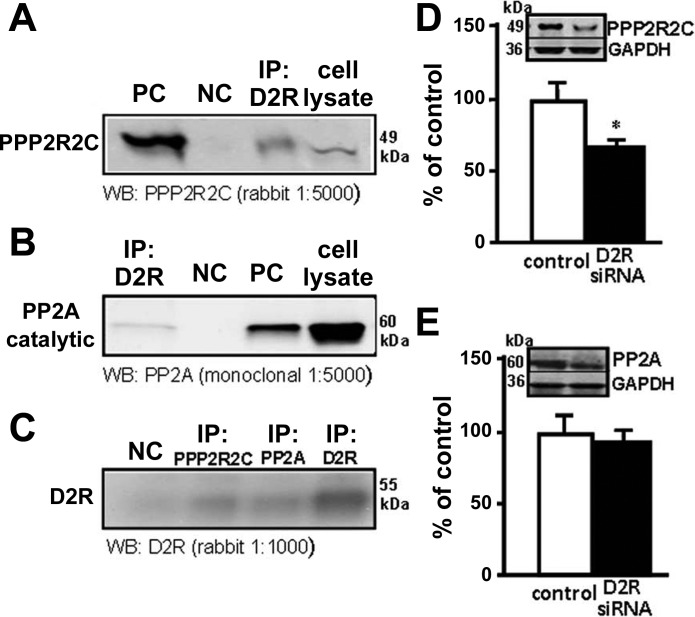
PP2A consists of a family of heterotrimers composed of one scaffolding (A), one catalytic (C), and one regulatory (B) subunit. There are several regulatory B subunits of which PPP2R2C, a γ-isoform of the subunit B55 subfamily, has been reported to associate with Akt selectively, regulating phosphorylation of Akt at Thr-308 (25). We evaluated the interaction between the D2R and PP2A catalytic subunit and PPP2R2C. Total mouse RPTC lysates were immunoprecipitated using a monoclonal anti-D2R antibody, and the eluates were subsequently immunoblotted with a PPP2R2C or a PP2A catalytic subunit antibody. As shown in Fig. 4A, one band was visualized, which corresponded to PPP2R2C, as a similar band was obtained when the whole cell lysate was immunoprecipitated with anti-PPP2R2C antibody. No coimmunoprecipitation was observed when normal rabbit IgG was used as the immunoprecipitant. Similarly, the PP2A catalytic subunit also coimmunoprecipitated with the D2R although to a lesser extent (Fig. 4B). Total cell lysates were also immunoprecipitated using anti D2R, PPP2R2C, and PP2A catalytic subunit antibodies and immunoblotted for the D2R. Both PPP2R2C and PP2A immunoprecipitated the D2R, but PP2A did it to a lesser extent. As shown by the bands obtained, ∼25% of the total D2R was immunoprecipitated by PPP2R2C but only <15% was immunoprecipitated by PP2A (Fig. 4C).
Fig. 4.
The D2R interacts with protein phosphatase 2A (PP2A). A: whole RPTC lysates were immunoprecipitated with anti-D2R antibody and immunostained with anti-PP2A regulatory subunit PPP2R2C. negative control (NC), mouse IgG; positive control (PC), anti-PPP2R2C and whole cell lysate. B: whole RPTC lysates were immunoprecipitated with anti-D2R antibody anti-PP2A catalytic subunit. NC: mouse IgG; PC, anti-PP2A catalytic subunit. C: whole cell lysates were immunoprecipitated with anti-D2R antibody, anti-PP2A catalytic subunit and anti-PPP2R2C, and immunoblotted with anti-D2R. NC, mouse IgG. These experiments were repeated 3 times with similar results. D and E: mouse RPTCs were transfected with NS siRNA or D2R siRNA for 72 h. Protein expression of PPP2R2C and PP2A catalytic subunit was quantified by immunoblotting. Inset: 1 set of immunoblots. Results are expressed as percentage of NS siRNA. *P < 0.05 vs. NS siRNA; n = 4/group.
We next assessed the effect of the D2R on the expression of PPP2R2C and the PP2A catalytic subunit by silencing the D2R in mouse RPTCs. We found that PPP2R2C expression was decreased in mouse RPTCs transfected with Drd2 siRNA for 72 h (Fig. 4D). However, silencing the D2R did not modify the expression of the PP2A catalytic subunit (Fig. 4E). These results may be taken to suggest that D2R and PPP2R2C physically and functionally interact in mouse RPTCs. Further experimental proof is necessary to confirm D2R and PPP2R2C physical interaction.
PPP2R2C silencing results in increased Akt phosphorylation and expression of proinflammatory factors.
Treatment of mouse RPTCs with PPP2R2C siRNA significantly decreased PPP2R2C expression (Fig. 5A). The expression of phosphorylated Akt (2.2-fold), GSK3β (2-fold), and cyclin D1(1.7-fold) was increased in mouse RPTCs transfected with PPP2R2C siRNA for 48 h compared with mouse RPTCs transfected with no-silencing siRNA (Fig. 5A), indicating that PPP2R2C is important in the dephosphorylation of Akt.
Fig. 5.
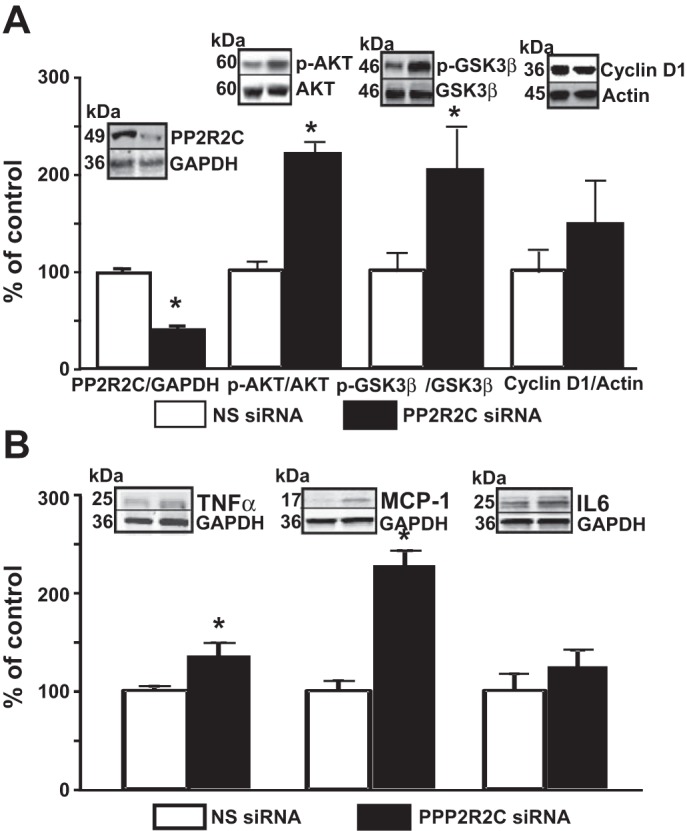
Silencing PPP2R2C increases Akt activity and proinflammatory factors. A and B: mouse RPTCs were transfected with NS siRNA and PPP2R2C siRNA. Protein expressions of D2R, Akt, p-Akt, GSK3β, p-GSK3β, and cyclin D1 (A) and TNF-α, MCP-1, and IL-6 (B) were quantified by immunoblotting. Inset: 1 set of immunnoblots Results are expressed as percentage of NS siRNA. *P < 0.05 vs. NS siRNA; n = 5/group.
We next determined whether PPP2R2C silencing and the associated increase in the Akt-GSK3β pathway reproduce the effects of D2R silencing on the expression of proinflammatory factors. The expression of TNF-α (1.4-fold), MCP-1 (2.1-fold), and IL-6 (1.3-fold) was increased in mouse RPTCs transfected with PPP2R2C siRNA for 48 h (Fig. 5B).
Increasing PP2A activity blunts the increase in NF-κb reporter activity induced by D2R silencing.
We have shown that in mouse RPTCs D2R silencing increases NF-κB reporter activity (56). To determine the role of PP2A in the increase in NF-κB reporter activity induced by D2R silencing, we used a PP2A activator (protamine, 5 μM) to increase PP2A activity. The stimulation of PP2A activity did not affect NF-κB reporter activity in cells treated with non-silencing siRNA but almost completely abolished the increase in the activity in cells with D2R downregulation (Fig. 6), indicating that for the most part the increase in NF-κB reporter activity was related to the decreased PP2A activity.
Fig. 6.
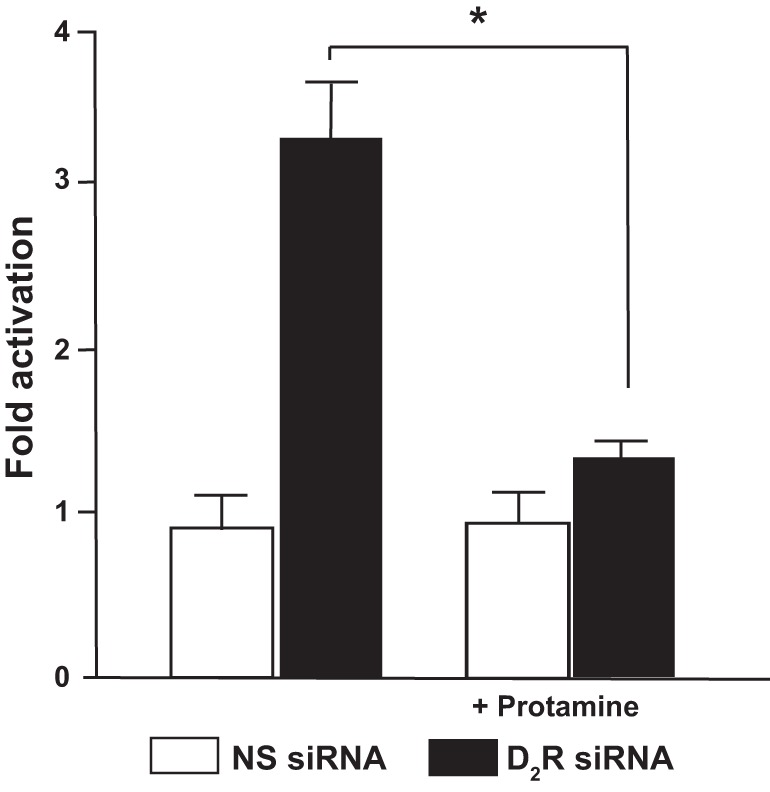
Increasing PP2A activity blunts the increase in NF-κB activity induced by D2R silencing. Mouse RPTCs were transfected with NS siRNA or D2R siRNA for 48 h and treated with vehicle or protamine (5 μM, PP2A activator). NF-κB activation was analyzed via the transient expression of an NF-κB-luciferase reporter system by reverse transfection. Results are expressed as fold-activation compared with vehicle treatment. *P < 0.05 vs. NS siRNA; n = 4/group.
DISCUSSION
The results of this study show that the protective effects of the D2R on renal production of inflammatory factors are exerted at least in part through enabling Akt dephosphorylation by PP2A. Decrease or lack of D2R function leads to enhanced Akt phosphorylation. Downregulation of the D2R is also associated with decreased expression of the PP2A regulatory subunit PPP2R2C that may physically interact with the D2R, indicating that the D2R is required for normal renal PP2A activity, termination of Akt signaling (Akt dephosphorylation), and inhibition of NF-κB (Fig. 7).
Fig. 7.
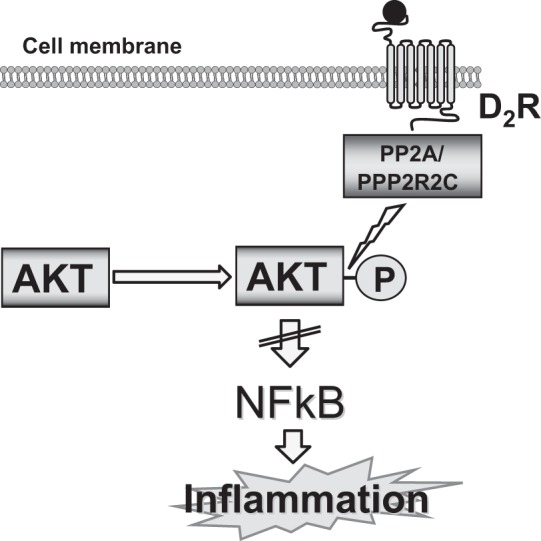
Schematic pathway of the protective effects of the D2R on renal inflammation. The protective effects of the D2R on renal production of inflammatory factors are exerted at least in part through enabling Akt dephosphorylation by PP2A. Decrease or lack of D2R function leads to enhanced Akt phosphorylation. Downregulation of the D2R is also associated with decreased expression of the PP2A regulatory subunit PPP2R2C that physically interacts with the D2R, indicating that the D2R is required for normal renal PP2A activity, termination of Akt signaling (Akt dephosphorylation), and inhibition of NF-κB.
The PI3K-Akt pathway is one of the pathways involved in the activation of NF-κB. Akt is activated by phosphorylation at its threonine (Thr 308) and serine (Ser 473) residues (35). Activated Akt activates IkB kinase, which phosphorylates IkB-α, the endogenous inhibitor of NF-κB, leading to the release and nuclear translocation of NF-κB (33). Activation and nuclear translocation of the transcription factor NF-κB are two of the mechanisms that regulate the expression of numerous genes related to inflammation such as cytokines/chemokines (27). NF-κB activation has been documented in vivo and in vitro in intrinsic glomerular cells, such as podocytes and mesangial, tubular, and endothelial cells, in renal injury or after exposure to inflammatory stimuli. Many stimuli activate canonical NF-κB in cultured renal cells to regulate the transcription of multiple proinflammatory molecules (16). In renal cells, NF-κB is activated by TNF-α and mediates the inflammatory response to TNF-α and IL-1 (37), generating a positive-feedback loop of activation (13).
Downregulation of the D2R in mouse RPTCs is associated with increased NF-κB activation and expression of proinflammatory factors that are dependent on NF-κB activity, consistent with increased Akt phosphorylation and activity, demonstrated by effects on downstream targets. Because both RPTCs and microdissected renal proximal tubules, in culture, produce significant amounts of dopamine from l-DOPA present in the serum added to the medium (20), the D2R in RPTCs is activated even in the absence of ligand added to the medium. The D2R may also be constitutively active (24). In the pituitary gland, D2R constitutively inhibits prolactin secretion (39).
Akt is dephosphorylated and inactivated by PP2A. PP2A is a multimeric phosphoprotein phosphatase composed of one scaffolding, one catalytic, and one regulatory (B) subunit (22, 30, 31, 48). The regulatory subunit determines subcellular localization, substrate specificity, signaling complexes, fine-tuning of enzyme activity, and controls the access of the substrate to the catalytic pocket of the catalytic subunit (22, 43, 48, 51). The diversity of PP2A regulatory subunits allows multiple patterns of assembly conferring spatial and temporal precision to PP2A-mediated dephosphorylation, and distinct cellular functions to different forms of PP2A (21, 43). Inactivation of Akt by dopamine can be prevented by inhibiting PP2A (4). In mouse RPTCs, inhibition of PP2A increased the phosphorylation of Akt and GSK3β, indicating that in these cells Akt activity is regulated by this phosphatase. Furthermore, D2R stimulation increased PP2A activity, suggesting that in renal cells D2R modulates the Akt-GSK3β pathway through regulation of PP2A activity. In the mouse striatum (4, 8, 18) and pituitary lactotrophs (35), rat frontal cortex (45), culture undifferentiated human neuroblastoma cells (14), gastric cancer cells (15), and zebrafish brain (44), D2R stimulation results in the inactivation of Akt. However, there are several reports of activation of the Akt-GSK3β pathway by D2R stimulation (9, 32, 36). Beaulieu et al. (6) have proposed that this apparent discrepancy may arise from the intrinsic temporal dynamics of the signaling mechanisms involved, with Akt inactivation mediated by β-arrestin occurring at periods later than responses mediated by G proteins and associated with Akt activation.
The D2R facilitates Akt inactivation by promoting the formation of a signaling complex with Akt, β-arrestin 2, and PP2A (4, 5, 7). Consistent with this proposed pathway, we found that D2R coimmunoprecipitated with both the catalytic and PPP2R2C regulatory subunits of PP2A. D2R downregulation also resulted in decreased PPP2R2C expression, presumably by increased degradation, but did not affect the expression of the catalytic subunit. Moreover, downregulation of PPP2R2C increased phosphorylated Akt and Akt-GSK3β pathway activity reproducing the effects of D2R silencing, suggesting that the effects of D2R downregulation on the expression of proinflammatory factors in mouse RPTCs are related to PP2A/PPP2R2C activity. It is possible that in the absence of the D2R the assembly of the signaling complex and consequently its function are impaired. Further evidence of the role of PP2A was provided by studying the effects of increasing PP2A activity on NF-κB promoter activity. As we have reported (56), D2R downregulation increases NF-κB promoter activity in mouse RPTCs. This increase is almost completely reversed when PP2A activity is increased, supporting the hypothesis that the protective effects of D2R on renal inflammation are mediated by PP2A/PPP2R2C regulation of Akt phosphorylation.
There is some evidence that the D2Rs have protective effects in organs other than the kidney. In the respiratory tract, D2R activation inhibits neurogenic inflammation (34). GLC756, a D1R antagonist/D2R agonist, inhibited TNF-α release from rat mast cells, suggesting that it reduced inflammation triggered by activated cells (26). In a mouse model of amyotrophic lateral sclerosis, a D2R agonist suppresses glial inflammation and moderates the progression of the disease (46). Lack of the D2R is associated with an inflammatory response in multiple central nervous system regions resulting from the hyperresponse of astrocytes to immune stimuli, indicating that astrocytic D2R activation normally suppresses neuroinflammation. The D2R modulation of the innate immune response in astrocytes is mediated by αB-crystalin and is also associated with increased GSK3β but not Akt phosphorylation (41).
Individuals carrying D2R polymorphisms that result in decreased expression of the D2R may be more vulnerable to renal injury (23) when challenged with an insult such as elevated blood pressure. The results of this study may provide the basis for targeted pharmacological treatment to ameliorate the insult, which should result in decreased prevalence of renal injury and chronic kidney disease.
GRANTS
The work was funded by National Institutes of Health Grants P01HL074940, P01HL068686, R01HL092196, R37HL023081, R01DK039308, and R01DK090918.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: Y.Z. and S.C. performed experiments; Y.Z. and S.C. analyzed data; Y.Z. and X.J. interpreted results of experiments; Y.Z., X.J., and S.C. prepared figures; Y.Z., X.J., C.Q., and S.C. drafted manuscript; C.Q., P.A.J., and I.A. edited and revised manuscript; C.Q., P.A.J., and I.A. approved final version of manuscript; I.A. provided conception and design of research.
REFERENCES
- 1.Armando I, Villar VA, Jose PA. Dopamine and renal function and blood pressure regulation. Compr Physiol 1: 1075–117, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Armando I, Wang X, Villar VA, Jones JE, Asico LD, Escano C, Jose PA. Reactive oxygen species-dependent hypertension in dopamine D2 receptor-deficient mice. Hypertension 49: 672–678, 2007. [DOI] [PubMed] [Google Scholar]
- 3.Asghar M, Chugh G, Lokhandwala MF. Inflammation compromises renal dopamine D1 receptor function in rats. Am J Physiol Renal Physiol 297: F1543–F1549, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell 122: 261–273, 2005. [DOI] [PubMed] [Google Scholar]
- 5.Beaulieu JM, Gainetdinov RR, Caron MG. The Akt-GSK-3 signaling cascade in the actions of dopamine. Trends Pharmacol Sci 28: 166–172, 2007. [DOI] [PubMed] [Google Scholar]
- 6.Beaulieu JM, Del'Guidice T, Sotnikova TD, Lemasson M, Gainetdinov RR. Beyond cAMP: the regulation of Akt and GSK3β by dopamine receptors. Front Mol Neurosci 4: 1–13, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beaulieu JM, Gainetdinov RR. The physiology, signaling and pharmacology of dopamine receptors. Pharm Rev 63: 182–217, 2011. [DOI] [PubMed] [Google Scholar]
- 8.Beaulieu JM, Sotnikova TD, Yao WD, Kockeritz L, Woodgett JR, Gainetdinov RR, Caron MG. Lithium antagonizes dopamine-dependent behaviors mediated by an AKT/glycogen synthase kinase 3 signaling cascade. Proc Natl Acad Sci USA 101: 5099–5104, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brami-Cherrier K, Valjent E, Garcia M, Pagès C, Hipskind RA, Caboche J. Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation. J Neurosci 22: 8911–8921, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen K, Iribarren P, Gong W, Wang JM. The essential role of phosphoinositide 3-kinases (PI3Ks) in regulating pro-inflammatory responses and the progression of cancer. Cell Mol Immunol 2: 241–252, 2005. [PubMed] [Google Scholar]
- 11.Chugh G, Lokhandwala MF, Asghar M. Oxidative stress alters renal D1 and AT1 receptor functions and increases blood pressure in old rats. Am J Physiol Renal Physiol 300: F133–F138, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cohen P1, Klumpp S, Schelling DL. An improved procedure for identifying and quantitating protein phosphatases in mammalian tissues. FEBS Lett 250: 596–600, 1989. [DOI] [PubMed] [Google Scholar]
- 13.Covert MW, Leung TH, Gaston Baltimore D JE. Achieving stability of lipopolysaccharide-induced NFkappa B activation. Science 309: 1954–1857, 2005. [DOI] [PubMed] [Google Scholar]
- 14.Deslauriers J1, Desmarais C, Sarret P, Grignon S. α-Lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3β signaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J Mol Neurosci 50: 134–145, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Ganguly S1, Basu B, Shome S, Jadhav T, Roy S, Majumdar J, Dasgupta PS, Basu S. Dopamine, by acting through its D2 receptor, inhibits insulin-like growth factor-I (IGF-I)-induced gastric cancer cell proliferation via up-regulation of Krüppel-like factor 4 through down-regulation of IGF-IR and AKT phosphorylation. Am J Pathol 177: 2701–2707, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guijarro C, Egido J. Transcription factor kappa B (NF-kappa B) and renal disease. Kidney Int 59: 415–424, 2001. [DOI] [PubMed] [Google Scholar]
- 17.Harrison DG, Guzik TJ, Lob HE, Madhur MS, Marvar PJ, Thabet SR, Vinh A, Weyand CM. Inflammation, immunity, and hypertension. Hypertension 57: 132–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harrison LM, Muller SH, Spano D. Effects of the Ras homolog Rhes on Akt/protein kinase B and glycogen synthase kinase 3 phosphorylation in striatum. Neuroscience 236: 21–30, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MM, Hemmings BA. Inhibition of protein kinase B/Akt. Implications for cancer therapy. Pharmacol Ther 93: 243–251, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Ibarra FR, Armando I, Nowicki S, Carranza A, Sarobe VDL, Arrizurieta EE, Barontini M. Dopamine is metabolized by different enzymes along the rat nephron. Pflügers Arch 450: 185–191, 2005. [DOI] [PubMed] [Google Scholar]
- 21.Janssens V, Goris J. Protein phosphatase 2A: a highly regulated family of serine/threonine phosphatases implicated in cell growth and signalling. Biochem J 353: 417–439, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janssens V, Longin S, Goris J. PP2A holoenzyme assembly: in cauda venenum (the sting is in the tail). Trends Biochem Sci 33: 113–121, 2008. [DOI] [PubMed] [Google Scholar]
- 23.Jiang X, Konkalmatt P, Yang Y, Gildea J, Jones JE, Cuevas S, Felder RA, Jose PA, Armando I. Single-nucleotide polymorphisms of the dopamine D2 receptor increase inflammation and fibrosis in human renal proximal tubule cells. Hypertension 63: e74–e80, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kozell LB, Neve KA. Constitutive activity of a chimeric D2/D1 dopamine receptor. Mol Pharmacol 52: 1137–1149, 1997. [DOI] [PubMed] [Google Scholar]
- 25.Kuo YC, Huang KY, Yang CH, Yang YS, Lee WY, Chiang CW. Regulation of phosphorylation of Thr-308 of Akt, cell proliferation, and survival by the B55alpha regulatory subunit targeting of the protein phosphatase 2A holoenzyme to Akt. J Biol Chem 283: 1882–1892, 2008. [DOI] [PubMed] [Google Scholar]
- 26.Laengle UW1, Markstein R, Pralet D, Seewald W, Roman D. Effect of GLC756, a novel mixed dopamine D1 receptor antagonist and dopamine D2 receptor agonist, on TNF-alpha release in vitro from activated rat mast cells. Exp Eye Res 83: 1335–1339, 2006. [DOI] [PubMed] [Google Scholar]
- 27.Li Q, Verma IM. NF-kappaB regulation in the immune system. Nat Rev Immunol 2: 725–734, 2002. [DOI] [PubMed] [Google Scholar]
- 28.Li XX, Bek M, Asico LD, Yang Z, Grandy DK, Goldstein DS, Rubinstein M, Eisner GM, Jose PA. Adrenergic and endothelin B receptor-dependent hypertension in dopamine receptor type-2 knockout mice. Hypertension 38: 303–308, 2001. [DOI] [PubMed] [Google Scholar]
- 29.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the ΔΔ CT method. Methods 25: 402–408, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Mumby M. PP2A: unveiling a reluctant tumor suppressor. Cell 130: 21–24, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Mumby M. The 3D structure of protein phosphatase 2A: new insights into a ubiquitous regulator of cell signaling. ACS Chem Biol 2: 99–103, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Nair VD, Sealfon SC. Agonist-specific transactivation of phosphoinositide 3-kinase signaling pathway mediated by the dopamine D2 receptor. J Biol Chem 278: 47053–47061, 2003. [DOI] [PubMed] [Google Scholar]
- 33.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature 401: 82–85, 1999. [DOI] [PubMed] [Google Scholar]
- 34.Peiser C1, Trevisani M, Groneberg DA, Dinh QT, Lencer D, Amadesi S, Maggiore B, Harrison S, Geppetti P, Fischer A. Dopamine type 2 receptor expression and function in rodent sensory neurons projecting to the airways. Am J Physiol Lung Cell Mol Physiol 289: L153–L158, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Radl D, De Mei C, Chen E, Lee H, Borrelli E. Each individual isoform of the dopamine D2 receptor protects from lactotroph hyperplasia. Mol Endocrinol 27: 953–965, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rau TF1, Kothiwal A, Zhang L, Ulatowski S, Jacobson S, Brooks DM, Cardozo-Pelaez F, Chopp M, Poulsen DJ. Low dose methamphetamine mediates neuroprotection through a PI3K-AKT pathway. Neuropharmacology 61: 677–686, 2011. [DOI] [PubMed] [Google Scholar]
- 37.Sanz AB, Sanchez-Nino MD, Ramos AM, Moreno JA, Santamaria B, Ruiz-Ortega M, Egido J, Ortiz A. NFkB in renal inflammation. J Am Soc Nephrol 21: 254–1262, 2010. [DOI] [PubMed] [Google Scholar]
- 38.Scheid MP, Woodgett JR. PKB/AKT: functional insights from genetic models. Nat Rev Mol Cell Biol 2: 760–768, 2001. [DOI] [PubMed] [Google Scholar]
- 39.Schuff KG, Hentges ST, Kelly MA, Binart N, Kelly PA, Iuvone PM, Asa SL, Low MJ. Lack of prolactin receptor signaling in mice results in lactotroph proliferation and prolactinomas by dopamine-dependent and -independent mechanisms. J Clin Invest 110: 973–981, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segerer S, Schlondorff D. Role of chemokines for the localization of leukocyte subsets in the kidney. Semin Nephrol 27: 260–274, 2007. [DOI] [PubMed] [Google Scholar]
- 41.Shao W1, Zhang SZ, Tang M, Zhang XH, Zhou Z, Yin YQ, Zhou QB, Huang YY, Liu YJ, Wawrousek E, Chen T, Li SB, Xu M, Zhou JN, Hu G, Zhou JW. Suppression of neuroinflammation by astrocytic dopamine D2 receptors via αB-crystallin. Nature 494: 90–94, 2013. [DOI] [PubMed] [Google Scholar]
- 42.Sontag E. Protein phosphatase 2A: the Trojan Horse of cellular signaling. Cell Signal 13: 7–16, 2001. [DOI] [PubMed] [Google Scholar]
- 43.Sontag E, Nunbhakdi-Craig V, Lee G, Bloom GS, Mumby MC. Regulation of the phosphorylation state and microtubule-binding activity of tau by protein phosphatase 2A. Neuron 17: 1201–1207, 1996. [DOI] [PubMed] [Google Scholar]
- 44.Souza BR, Romano-Silva MA, Tropepe V. Dopamine D2 Receptor activity modulates Akt signaling and alters GABAergic neuron development and motor behavior in zebrafish larvae. J Neurosci 31: 5512–5525, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sutton LP, Rushlow J. The dopamine D2 receptor regulates Akt and GSK-3 via Dvl-3. Int J Neuropsychopharmacol 22: 1–15, 2011. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka K1, Kanno T, Yanagisawa Y, Yasutake K, Hadano S, Yoshii F, Ikeda JE. Bromocriptine methylate suppresses glial inflammation and moderates disease progression in a mouse model of amyotrophic lateral sclerosis. Exp Neurol 232: 41–52, 2011. [DOI] [PubMed] [Google Scholar]
- 47.Ugi S, Imamura T, Maegawa H, Egawa K, Yoshizaki T, Shi K, Obata T, Ebina Y, Kashiwagi A, Olefsky JM. Protein phosphatase 2A negatively regulates insulin's metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol Cell Biol 24: 8778–8789, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Virshup DM, Shenolikar S:. From promiscuity to precision: protein phosphatases get a makeover. Mol Cell 33: 537–545, 2009. [DOI] [PubMed] [Google Scholar]
- 49.Wang Y, Tay YC, Harris DC. Proximal tubule cells stimulated by lipopolysaccharide inhibit macrophage activation. Kidney Int 66: 655–662, 2004. [DOI] [PubMed] [Google Scholar]
- 50.Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, Coffman TM, Hopfer U. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim 42: 189–200, 2006. [DOI] [PubMed] [Google Scholar]
- 51.Xu Y, Xing Y, Chen Y, Chao Y, Lin Z, Fan E, Yu JW, Strack S, Jeffrey PD, Shi Y. Structure of the protein phosphatase 2A holoenzyme. Cell 127: 1239–1251, 2006. [DOI] [PubMed] [Google Scholar]
- 52.Yang S, Yao B, Zhou Y, Yin H, Zhang MZ, Harris RC. Intrarenal dopamine modulates progressive angiotensin II-mediated renal injury. Am J Physiol Renal Physiol 302: F742–F749, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y, Zhang Y, Cuevas S, Villar VA, Escano C, Asico L D, Yu P, Grandy DK, Felder RA, Armando I, Jose PA. Paraoxonase 2 decreases renal reactive oxygen species production, lowers blood pressure, and mediates dopamine D2 receptor-induced inhibition of NADPH oxidase. Free Radic Biol Med 53: 437–446, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu P, Asico LD, Eisner GM, Hopfer U, Felder RA, Jose PA:. Renal protein phosphatase 2A. Activity, and spontaneous hypertension in rats. Hypertension 36: 1053–1058, 2000. [DOI] [PubMed] [Google Scholar]
- 55.Zhang MZ, Yao B, Wang S, Fan X, Wu G, Yang H, Yin H, Yang S, Harris RC. Intrarenal dopamine deficiency leads to hypertension and decreased longevity in mice. J Clin Invest 121: 2845–2854, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhang Y, Cuevas S, Asico LD, Escano C, Yang Y, Pascua AM, Wang X, Jones JE, Grandy D, Eisner G, Jose PA, Armando I. Deficient dopamine D2 receptor function causes renal inflammation independently of high blood pressure. PLoS One 7: e38745, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]