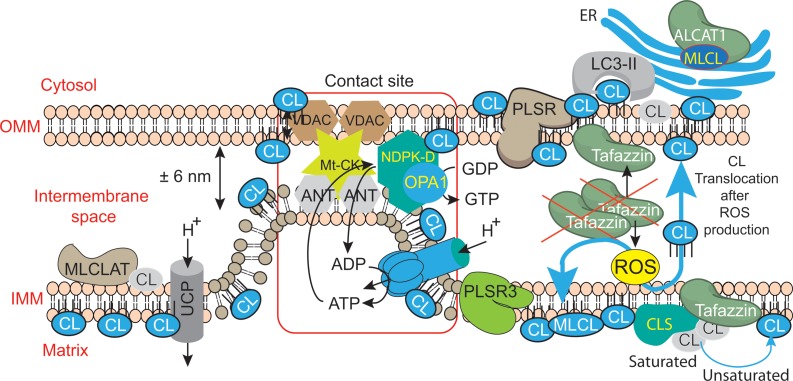
FIGURE 6.
Mitochondrial dysfunctions due to tafazzin mutations lead to ROS production, CL translocation and dysregulation of autophagic signaling. CL is synthesized in the inner leaflet of the IMM by cardiolipin synthase (CLS). This is also the localization where it can be remodeled by tafazzin and monolysocardiolipin acyltransferase (MLCLAT), in a balanced system with PLA2. During processing or following stress signaling, CLs may also be translocated to the OMM and ER for remodeling, whereas in healthy mitochondria most of the CL remains at its site of synthesis in the IMM. The externalization of mature CL to the mitochondrial surface, through a mechanism involving phospholipid scramblase-3 (PLSR3), is a prerequisite for its recognition by light chain 3 protein (LC3) as a mitophagic “eat-me signal” targeting dysfunctional mitochondria to the autophagosomal machinery. The precise mechanism of scramblase regulation remains unclear, but its action may be physically facilitated by the proximity of nucleoside diphosphate kinase (NDPK-D). NDPK-D regulates the balance between di- and tri-phosphonucleotides, which provide GTP for the GTPase activity of a dynamin-like protein encoded by the gene OPA1 (for optic atrophy gene 1). By forming a hexamer in the intermembrane space, NDPK-D forms a physical bridge between the IMM and OMM. The switch from the phosphotransfer mode to a CL-translocating mode would thus facilitate the redistribution of CLs between the IMM and OMM. Note that immature CLs containing saturated acyl chains are gray while mature CLs with unsaturated acyl chains are blue. [Figure reproduced with modifications from Kagan et al. (2014), with the permission of the authors and of Elsevier Press].