Abstract
Sphingosine-1-phosphate (S1P) is a bioactive lipid mediator that regulates many processes in inflammation and cancer. S1P is a ligand for five G-protein-coupled receptors, S1PR1 to -5, and also has important intracellular actions. Previously, we showed that intracellular S1P is involved in tumor necrosis factor alpha (TNF)-induced NF-κB activation in melanoma cell lines that express filamin A (FLNA). Here, we show that extracellular S1P activates NF-κB only in melanoma cells that lack FLNA. In these cells, S1P, but not TNF, promotes IκB kinase (IKK) and p65 phosphorylation, IκBα degradation, p65 nuclear translocation, and NF-κB reporter activity. NF-κB activation induced by S1P was mediated via S1PR1 and S1PR2. Exogenous S1P enhanced the phosphorylation of protein kinase Cδ (PKCδ), and its downregulation reduced S1P-induced the phosphorylation of IKK and p65. In addition, silencing of Bcl10 also inhibited S1P-induced IKK phosphorylation. Surprisingly, S1P reduced Akt activation in melanoma cells that express FLNA, whereas in the absence of FLNA, high phosphorylation levels of Akt were maintained, enabling S1P-mediated NF-κB signaling. In accord, inhibition of Akt suppressed S1P-mediated IKK and p65 phosphorylation and degradation of IκBα. Hence, these results support a negative role of FLNA in S1P-mediated NF-κB activation in melanoma cells through modulation of Akt.
INTRODUCTION
Sphingosine-1-phosphate (S1P) is a bioactive sphingolipid metabolite that regulates a myriad of physiological processes, including cell growth, survival, migration, and differentiation. S1P plays important roles in disorders of the immune and cardiovascular systems as well as in cancer (1–3). Most of the actions of S1P are mediated by binding to five specific S1P receptors, named S1PR1 to -5 (4, 5). These receptors are coupled to distinct heterotrimeric G proteins leading to downstream activation of diverse effector pathways, including phospholipase C (PLC), phosphatidylinositol 3-kinase (PI3K), and mitogen-activated protein kinases (MAPKs), among others (6).
S1P produced inside cells by the activation of two sphingosine kinases, SphK1 and SphK2 (3, 4), can be exported by either the specific transporter Spns2 (7) or several members of the ABC transporter family (8). S1P then acts in an autocrine or paracrine manner by a process coined “inside-out signaling” (3, 4). In this regard, we previously showed that the actin cross-linking protein filamin A (FLNA) is involved in inside-out signaling of S1P by linking SphK1 and S1PR1 at the leading edge of melanoma cells to promote cell movement (9). In addition, FLNA also associates with multiple noncytoskeletal proteins with diverse functions and provides a scaffold for a wide range of cytoplasmic and nuclear signaling proteins (10). For example, FLNA interacts with tumor necrosis factor (TNF) receptor-associated factor 2 (TRAF2) to promote the activation of NF-κB in melanoma cells (11). Interestingly, SphK1 binds both TRAF2 and FLNA, suggesting that the production of S1P has an important role in NF-κB signaling (9, 12). Indeed, we have recently shown that S1P formed intracellularly by TNF-mediated activation of SphK1 binds to and is a required cofactor for the E3 ubiquitin ligase activity of TRAF2, a key step in the NF-κB pathway (13). On the other hand, S1P also activates NF-κB by binding to specific S1PRs (14–16). However, the signaling pathways downstream of S1PRs leading to the activation of NF-κB are not fully understood. Thus, in the present work, we evaluated how extracellular S1P activates NF-κB and the role of FLNA in this mechanism.
MATERIALS AND METHODS
Reagents.
S1P was obtained from Enzo Life Sciences (Farmingdale, NY), and TNF-α was obtained from Roche (Hague Road, IN). JTE013 (S1PR2 antagonist) and VPC23019 (S1PR1/3 antagonist) were obtained from Avanti Polar Lipids (Alabaster, AL). W146 (S1PR1 antagonist), CAY10444 (S1PR3 antagonist), and SEW2871 (S1PR1 agonist) were obtained from Cayman Chemical (Ann Arbor, MI). CYM-5520 (S1PR2 agonist), phorbol 13-myristate 12-acetate (PMA) (diacylglycerol [DAG]-dependent protein kinase C [PKC] activator), Go6983 (PKC inhibitor), and rottlerin (PKCδ inhibitor) were obtained from Sigma (St. Louis, MO). Primary antibodies directed against phospho-p65 (S536), phospho-IκB kinase α/β (IKKα/β) (S176/180), phospho-IκBα (S32/36), total IκBα (mouse monoclonal antibody [MAb] L35A5), phospho-Akt (S473), phospho-PKC, phospho-STAT3 (Tyr705), and Akt were obtained from Cell Signaling (Beverly, MA). Extracellular signal-regulated kinase 1/2 (ERK1/2) (T202/Y204) and β-tubulin antibodies were obtained from Santa Cruz Biotechnology (Santa Cruz, CA). FLNA antibody was obtained from Abgent (San Diego, CA). S1PR1, S1PR2, and S1PR3 antibodies were obtained from Abcam (Cambridge, MA). Appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). Oligofectamine transfection reagent was purchased from Invitrogen (Carlsbad, CA). Small interfering RNAs (siRNAs) for SphK1, Bcl10, and PKCδ and control siRNA (siControl) were obtained from Qiagen (Valencia, CA), and human FLNA siRNA was obtained from Thermo Scientific Dharmacon (Lafayette, CO).
Cell culture.
M2 and A7 melanoma cells were cultured in minimal essential medium (MEM) (Gibco, USA) supplemented with 10% fetal bovine serum as described previously (9). M2 and A7 are a matched pair of cell lines: M2 cells are parental cells that do not express detectable levels of FLNA, while A7 cells are derived from M2 cells and stably express FLNA at near-normal levels (17). A7 cells were also cultured in the presence of 0.5 mg/ml G418. Lu1205 BRAFV600E (mutant), Sk-mel2 BRAFwt (wild-type), WM35, and FM16 melanoma cells were cultured in Dulbecco's modified Eagle's medium (DMEM). SH-SY5Y human neuroblastoma cells were cultured in DMEM supplemented with 10% fetal bovine serum. For experiments, cells plated into 6- or 12-well plates were cultured for 48 h at 37°C and serum starved overnight. Cells were treated with 100 nM S1P in serum-free MEM plus 4 mg/ml bovine serum albumin (BSA) (fatty acid free and gamma-globulin free; Sigma), 10 ng/ml TNF, or 200 nM PMA unless indicated otherwise. Cells were preincubated for 30 min with inhibitors or S1PR antagonists or agonists, as indicated in the figure legends.
For siRNA transfections, cells were plated into six-well plates at a density of 80,000 cells/well and transfected with Oligofectamine and a specific siRNA or siControl, as previously described (13). Downregulation of expression was confirmed by immunoblotting and/or quantitative PCR (QPCR).
Quantitative real-time PCR.
Total RNA was isolated with TRIzol (Invitrogen, Carlsbad, CA) and reverse transcribed with the High Capacity cDNA reverse transcription kit, premixed primer-probe sets, and TaqMan Universal PCR master mix (Applied Biosystems, USA). The cDNAs were diluted 10-fold (for the target genes) and amplified by using the ABI 7900HT cycler. Gene expression levels were normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) levels and are shown as mean values ± standard deviations (SD).
Western blotting.
Cells were washed with ice-cold phosphate-buffered saline (PBS) and scraped into lysis buffer containing 20 mM Tris-HCl (pH 7.4), 1 mM EDTA, 150 mM NaCl, 1% Triton X-100, 1 mM β-mercaptoethanol, 1 mM Na3VO4, and a 1:500 dilution of a protease inhibitor cocktail (Cayman, Ann Arbor, MI). Lysates were incubated on ice for 1 h and centrifuged at 10,000 × g for 10 min. Proteins (30 μg) were separated by SDS-PAGE and blotted onto nitrocellulose. Membranes were cut between high- and low-molecular-weight markers and incubated overnight at 4°C with specific primary antibodies (1:1,000). Immunopositive bands were visualized by enhanced chemiluminescence using secondary antibodies conjugated with horseradish peroxidase (1:10,000) at room temperature for 2 h and Super-Signal West Pico chemiluminescent substrate (Pierce Chemical Co., Rockford, IL). Blots were stripped and developed with antitubulin as a loading control. Optical densities of bands associated with proteins of interest were quantified by using Image Studio Lite (Li-Cor Biosciences, Lincoln, NE) or AlphaEaseFC software (Alpha Innotech, Miami, FL) and normalized to the optical densities of their respective tubulin bands.
Nuclear extracts.
Cells were washed with cold PBS; resuspended in buffer containing 10 mM HEPES (pH 7.8), 10 mM KCl, 0.1 mM EDTA, 1 mM Na3VO4, 1 mM dithiothreitol (DTT), and a 1:500 dilution of protease inhibitors (Sigma); and incubated on ice for 15 min. NP-40 was added to 0.75% (vol/vol), and cells were vortexed for 10 s. Nuclei and the supernatant (“cytoplasm”) were separated by centrifugation at 1,000 × g for 3 min at 4°C. Nuclei were resuspended in buffer containing 20 mM HEPES (pH 7.8), 0.4 M NaCl, 1 mM EDTA, 1 mM Na3VO4, 1 mM DTT, and a 1:500 dilution of protease inhibitors and incubated on ice for 15 min. Nuclear extracts were cleared by centrifugation at 14,000 × g for 5 min at 4°C.
NF-κB reporter assay.
M2 and A7 cells were transiently transfected with pNF-κB-Luc and pRSV-β-galactosidase. Forty-eight hours after transfection, cells were stimulated with TNF and S1P, and luciferase and β-galactosidase activities were determined with the Dual-Light chemiluminescent reporter gene assay (Tropix USA). Luciferase activity was normalized to β-galactosidase activity as described previously (13).
Electrophoretic mobility shift assays.
Electrophoretic mobility shift assays (EMSAs) were performed with 5 μg of nuclear protein and [α-32P]dCTP-end-labeled double-strand oligonucleotides containing an NF-κB consensus binding site (10 fmol; 10,000 cpm), as described previously (13).
Apoptosis assay.
Apoptosis was determined with the Cell Death Detection ELISAPlus kit (Roche) according to the manufacturer's protocols. Briefly, this assay measures mono- and oligonucleosomes in apoptotic-cell lysates. Lysates in streptavidin-coated microplates were incubated with antihistone/biotin and anti-DNA/peroxidase. Peroxidase activity in the immunocomplexes was measured at 405 nm after incubation with 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid).
Immunofluorescence.
Cells grown on glass coverslips and treated as indicated were fixed with 4% paraformaldehyde for 10 min, washed with 10 mM glycine in PBS to quench fixation, and permeabilized with 0.5% Triton X-100 for 2 min. After incubation with anti-p65 antibody (Santa Cruz Biotechnology) in 10 mg/ml BSA in PBS-glycine for 20 min, the coverslips were washed and then incubated for 10 min with Alexa 488–anti-rabbit secondary antibody (Invitrogen) in 10 mg/ml BSA in PBS containing 8 mg/ml Hoechst dye. Coverslips were mounted onto slides with 10 mM n-propylgallate in 100% glycerol and visualized on a Zeiss LSM 700 laser confocal microscope (13).
Statistical analysis.
Statistical analysis was performed by using unpaired two-tailed Student's t test for comparison of two groups and one-way analysis of variance (ANOVA) with Bonferroni post hoc comparison for experiments consisting of three or more groups (Prism; GraphPad Software, La Jolla, CA). A P value of <0.01 was considered significant. Experiments were repeated at least three times, with consistent results.
RESULTS
S1P activates NF-κB only in melanoma cells lacking FLNA.
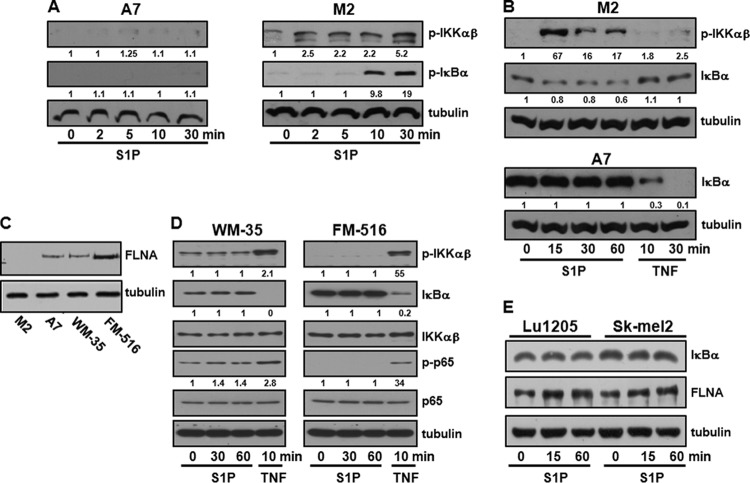
We have previously shown that intracellular S1P is required for TNF-induced NF-κB activation in A7 melanoma cells (13) that stably express FLNA at near-normal levels (17). However, in agreement with the results of our previous study (13), treatment of these cells with 100 nM exogenous S1P, a concentration that activates signaling pathways downstream of S1PRs, did not increase the phosphorylation of IKK, an enzyme complex containing two highly homologous kinase subunits, IKKα and IKKβ, that phosphorylate IκBα, leading to its proteasomal degradation (Fig. 1A, left). In contrast, as was reported previously (11), although TNF induced robust IκBα degradation in A7 cells, it had no effect on M2 cells that do not express FLNA (Fig. 1B). Similarly, TNF, but not 100 nM S1P, stimulated the phosphorylation of IKK and IκBα degradation in other melanoma cells that express FLNA, including weakly aggressive WM35 cells and the immortalized normal human melanocyte line FM516 (Fig. 1C and D). Likewise, S1P did not induce IκBα degradation in FLNA-expressing Sk-mel2 and Lu1205 cells (Fig. 1E). Remarkably, however, in the course of these experiments, we observed that in FLNA-deficient M2 melanoma cells, exogenous S1P (100 nM) was able to stimulate IKK phosphorylation and IκBα phosphorylation and degradation (Fig. 1A to C). S1P at this concentration activated ERK1/2 in M2 cells as well as in their FLNA counterpart A7 cells (data not shown), indicating that the ability of S1P to signal through its cell surface receptors is not impaired.
FIG 1.
S1P activates IKK and degradation of IκBα only in M2 melanoma cells deficient in FLNA. M2 and A7 melanoma cells (A to C), WM35 and FM516 melanoma cells (C and D), and Lu1205 and Sk-mel2 melanoma cells (E) were serum starved overnight and then treated with the vehicle, S1P (100 nM), or TNF (10 ng/ml) for the indicated times. Equal amounts of cell lysates were separated by SDS-PAGE and immunoblotted with the indicated antibodies. Blots were stripped and reprobed for tubulin to ensure equal loading and transfer. Numbers indicate relative protein levels determined by densitometry. Blots are representative of results from at least three independent experiments.
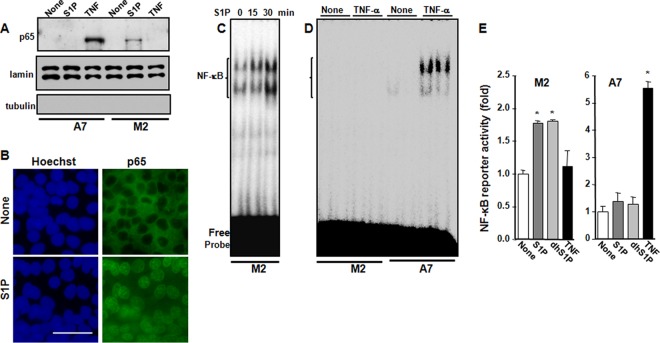
S1P induces p65 nuclear translocation and enhances NF-κB DNA binding and reporter activity only in M2 melanoma cells.
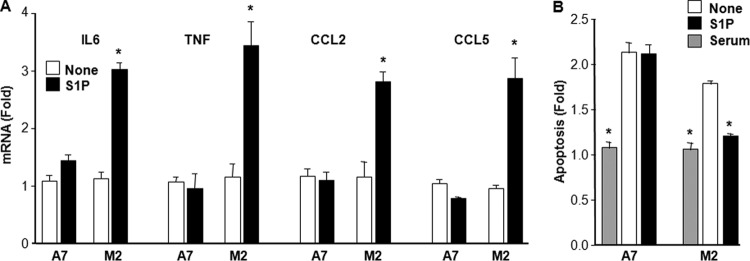
Following IκBα degradation, the transcription factor NF-κB composed of p65 and p50 subunits translocates to the nucleus and regulates gene transcription. As shown in Fig. 2A, TNF induces the translocation of p65 to the nucleus in A7 cells but not in M2 cells. Conversely, S1P induced the translocation of p65 to the nucleus within 30 min only in M2 cells but not in A7 cells. Furthermore, confocal microscopy also revealed that 100 nM S1P induced significant translocation of p65 to the nucleus in M2 cells (Fig. 2B). S1P also increased the DNA binding activity of NF-κB in M2 cells, as evident in EMSAs (Fig. 2C). In contrast, consistent with data from a previous report (11), TNF did not increase NF-κB DNA binding activity in M2 cells but readily increased it in A7 cells (Fig. 2D). To further confirm these results, M2 and A7 cells were transfected with an NF-κB luciferase reporter and then stimulated with S1P or TNF. NF-κB reporter activity was significantly increased by S1P or dihydro-S1P, another ligand that binds all of the S1PRs, only in FLNA-lacking M2 cells, while no effects were observed in A7 cells (Fig. 2E). Conversely, TNF increased NF-κB luciferase reporter activity only in FLNA-expressing A7 cells but not in M2 cells (Fig. 2E). Altogether, these results suggest that expression of FLNA prevents NF-κB activation induced by extracellular S1P. In agreement, S1P increased the expression levels of the NF-κB-regulated inflammatory cytokines interleukin-6 (IL-6) and TNF and the chemokines CCL2 and CCL5 only in M2 but not in A7 cells (Fig. 3A). Moreover, S1P-stimulated activation of NF-κB in M2 cells correlated with S1P suppression of serum withdrawal-induced apoptosis determined by measurement of mono- and oligonucleosomes in lysates of apoptotic cells (Fig. 3B). In contrast, S1P did not protect A7 cells from apoptosis.
FIG 2.
S1P promotes p65 translocation, NF-κB DNA binding, and reporter activity in FLNA-deficient M2 melanoma cells. (A) M2 and A7 cells were serum starved overnight and then stimulated with S1P (100 nM) or TNF (10 ng/ml) for 30 min. Nuclear fractions were prepared, and equal amounts of protein were analyzed by immunoblotting for p65. Lamin A/C was used as a nuclear marker, and tubulin was used as a cytosol marker. (B) M2 cells were serum starved overnight and then stimulated with S1P for 30 min. Cells were stained with Hoechst dye (blue) or p65 antibody (green) and visualized by confocal microscopy. Bar, 50 μm. (C and D) M2 and A7 cells were treated with S1P (100 nM) for the indicated times (C) or with TNF (10 ng/ml) for 30 min (D). NF-κB DNA binding activity in nuclear fractions (5 μg) was determined by EMSAs. (E) M2 and A7 cells were cotransfected with pNF-κB luciferase and β-galactosidase plasmids and then stimulated with S1P (100 nM), dihydro-S1P (dhS1P) (100 nM), or TNF (10 ng/ml) for 18 h. Luciferase activity was normalized to β-galactosidase activity and measured with the Dual-Light reporter gene assay. Data are expressed as fold increases and are means ± standard errors of the means from three independent experiments. *, P < 0.01 compared to no treatment.
FIG 3.
S1P stimulates expression of NF-κB-regulated cytokines and chemokines and protects FLNA-deficient M2 melanoma cells from serum-withdrawal-induced apoptosis. M2 and A7 cells were serum starved overnight and then treated for 24 h without or with S1P (100 nM) or 10% serum, as indicated. (A) IL-6, TNF, CCL2, and CCL5 mRNA levels were quantified by QPCR and normalized to GAPDH levels. (B) Apoptosis was analyzed by using the Cell Death Detection ELISAPlus kit. Data are means ± SD. *, P < 0.001, compared to untreated cells. Similar results were obtained in three independent experiments.
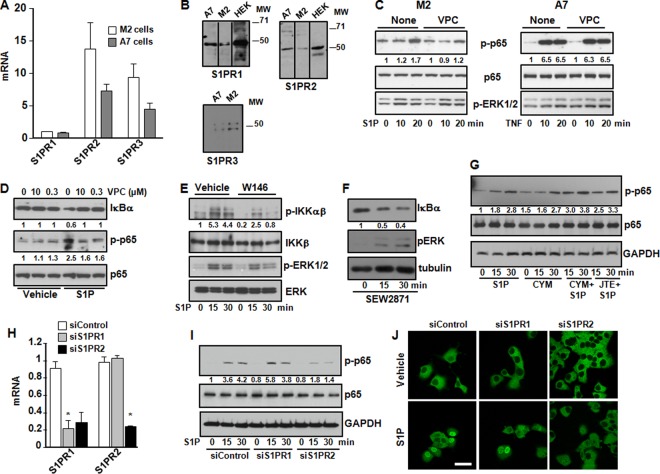
S1PR1 and S1PR2 are involved in activation of NF-κB by S1P.
Next, we determined which of the S1PRs are involved in S1P-induced NF-κB activation. As assessed by QPCR, both M2 and A7 cells express S1PR1 to -3, while no message was detected for S1PR4 or S1PR5 (Fig. 4A). Western blotting also confirmed the expression of S1PR1 to -3 in both cell lines (Fig. 4B). The specificity of the S1PR1 and S1PR2 antibodies was confirmed with HEK293 cells overexpressing these receptors (Fig. 4B). To evaluate which S1PR subtype is involved in NF-κB activation, we first utilized specific S1PR antagonists and examined their effects on p65 serine 536 phosphorylation that is important for p65 nuclear import (18). VPC23019 is an antagonist of S1PR1 and S1PR3 at a high concentration (10 μM) but is an S1PR1 antagonist only at a low concentration (0.3 μM) (19). VPC23019 decreased S1P- but not TNF-induced p65 phosphorylation in M2 and A7 cells, respectively (Fig. 4C). In addition, VPC23019 suppressed S1P-induced IκBα degradation and p65 phosphorylation at a low concentration as effectively as at a high concentration, suggesting the involvement of S1PR1 (Fig. 4D). In agreement, W146, a S1PR1-selective competitive antagonist, ablated S1P-stimulated IKK phosphorylation, although it had only a small effect on ERK1/2 phosphorylation (Fig. 4E). Furthermore, the specific S1PR1 agonist SEW2871 also induced IκBα degradation (Fig. 4F). Although the specific S1PR2 antagonist JTE013 did not affect S1P-induced phosphorylation of p65 (Fig. 4G), surprisingly, CYM-5520, a noncompetitive allosteric agonist of S1PR2, also stimulated the phosphorylation of p65 similarly to S1P (Fig. 4G). Therefore, it was of interest to examine the effects of downregulation of S1PR1 and S1PR2. siRNA targeted to S1PR1, which decreased its expression by 77% (Fig. 4H), had no significant effect on S1P-stimulated p65 phosphorylation (Fig. 4I) or nuclear translocation (Fig. 4J). However, transfection of M2 cells with siRNA targeted to S1PR2, which reduced its expression by >75% and also somewhat unexpectedly reduced S1PR1 expression by 70% (Fig. 4H), markedly suppressed S1P-induced p65 phosphorylation (Fig. 4I) as well as its nuclear translocation (Fig. 4J). These results suggest that in FLNA-deficient M2 melanoma cells, both S1PR1 and S1PR2 are involved in the activation of NF-κB by S1P.
FIG 4.
S1P activates NF-κB via S1PR1 and S1PR2. (A and B) M2 and A7 melanoma cells were serum starved overnight. (A) RNA was isolated, and levels of S1PR1 to -3 mRNAs were quantified by QPCR and normalized to GAPDH levels. Data are expressed as relative expression levels and are means ± standard errors of the means from three independent experiments. (B) Cell lysates were analyzed by immunoblotting with S1PR-specific antibodies, and tubulin was used as a loading control. HEK293 cells overexpressing S1PR1 or S1PR2 were used to confirm antibody specificities. MW, molecular weight (in thousands). (C) M2 and A7 cells were serum starved overnight, pretreated with VPC23019 (VPC) (10 μM) for 30 min, and then stimulated without or with S1P (100 nM) or TNF (10 ng/ml) for the indicated times. (D to F) M2 cells were serum starved overnight; pretreated for 30 min with VPC23019 (0.3 or 10 μM) (D), W146 (10 μM) (E), or SEW2871 (10 μM) (F); and then stimulated without or with S1P (100 nM) for 30 min or the indicated times. (G) M2 cells were serum starved overnight; treated with S1P (100 nM), CYM-5520 (CYM) (1 μM), or both or pretreated with JTE013 (JTE) (10 μM) for 30 min; and then stimulated with S1P (100 nM), as indicated. In panels C to G, cell lysates were analyzed by immunoblotting with the indicated antibodies. Numbers indicate relative protein levels determined by densitometry. (H to J) M2 cells were transfected with siControl, siS1PR1, or siS1PR2, as indicated. (H) mRNA levels were determined by QPCR and normalized to GAPDH levels. Data are expressed as relative expression levels compared to those for siControl and are means ± SD from three independent experiments. *, P < 0.001. (I and J) Cells were treated without or with S1P (100 nM). In panel I, cell lysates were analyzed by immunoblotting with the indicated antibodies. Numbers indicate relative protein levels determined by densitometry. (J) Cells were stained with anti-p65 antibody and visualized by confocal microscopy. Blots are representative of results from three independent experiments.
Activation of NF-κB by S1P requires PKCδ and involves Bcl10.
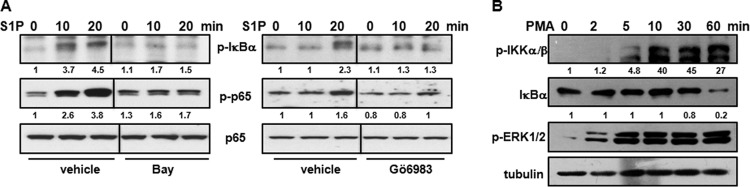
It was important to examine the intracellular signaling pathways downstream of S1PR engagement leading to the activation of NF-κB. Because previous studies have shown that PKC is involved in NF-κB activation induced by lysophosphatidic acid (LPA) (20, 21), another lysophospholipid that signals through a family of G-protein-coupled receptors (GPCRs) related to S1PRs (5), we determined whether PKC activity was needed for S1P-induced NF-κB activation. First, pretreatment of M2 cells with the broad-spectrum PKC inhibitor Go6983 decreased the phosphorylation of IκBα and p65 induced by S1P to a similar extent as the IKK inhibitor Bay 11-7082 (Fig. 5A). Indeed, direct stimulation of PKC with phorbol 12-myristate 13-acetate (PMA) markedly stimulated IKK phosphorylation and increased IκBα degradation in a time-dependent manner in M2 cells (Fig. 5B).
FIG 5.
S1P-induced activation of NF-κB involves PKC. (A) M2 cells were serum starved overnight, preincubated with 5 μM Bay 11-7082 (Bay) or 5 μM Go6983, and stimulated with S1P (100 nM) for the indicated times. (B) M2 cells were serum starved overnight and stimulated with PMA (100 nM) for the indicated times. Cell lysates were analyzed by immunoblotting with the indicated antibodies. Blots were stripped and reprobed with p65 (A) or tubulin (B) to ensure equal loading and transfer. Blots are representative of results from three (A) and two (B) independent experiments. Numbers indicate relative protein levels determined by densitometry.
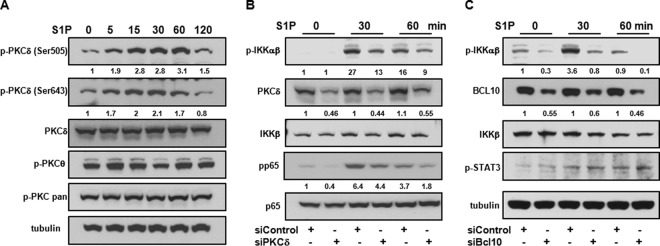
Second, to identify the PKC isoform involved in S1P action, we utilized phospho-PKC antibodies to evaluate the phosphorylation status of specific PKC isoforms. Surprisingly, S1P did not enhance the phosphorylation observed with pan-phospho-PKC antibody, which detects phosphorylation only at a carboxy-terminal residue of the PKC α, βI, βII, δ, ε, and η isoforms (homologous to Ser660 of PKCβ) (Fig. 6A), nor did it increase the phosphorylation of PKCθ at Ser676 (Fig. 6A). However, a significant increase in the level of phosphorylated PKCδ at Thr505 in the activation loop and Ser643 residues that are important for the activation of PKCδ (22) was clearly evident within 5 min after stimulation with S1P, and phosphorylation continued for at least 60 min (Fig. 6A).
FIG 6.
PKCδ and Bcl10 are involved in S1P-mediated NF-κB activation. (A) M2 cells were serum starved overnight and stimulated with S1P (100 nM) for the indicated times. (B and C) M2 cells were transfected with control siRNA or siRNA directed against PKCδ (B) or Bcl10 (C). After 48 h, cells were serum starved overnight and stimulated with S1P (100 nM). Cell lysate proteins were analyzed by immunoblotting with the indicated antibodies. Tubulin (A and C) or p65 (B) was used to ensure equal loading and transfer. Blots are representative of results from two independent experiments. Numbers indicate relative protein levels determined by densitometry.
Finally, to confirm the involvement of PKCδ in S1P-induced activation of NF-κB, PKCδ expression in M2 cells was silenced with a specific siRNA that significantly decreased its expression by 50% ± 5% (Fig. 6B). Downregulation of PKCδ reduced S1P-mediated phosphorylation of IKK and p65 compared to siControl (Fig. 6B). Hence, pharmacological and molecular approaches demonstrate that PKCδ contributes to S1P-induced activation of NF-κB in melanoma cells lacking FLNA.
As there is increasing evidence that the adaptor protein Bcl10 (B-cell lymphoma 10) is a key signaling component in PKC-mediated IKK activation induced by the ligation of LPA receptors (21, 23), the requirement for Bcl10 was then examined. Similar to the effect of siRNA for PKCδ (siPKCδ), siRNA that diminished Bcl10 expression by 45% ± 9% reduced S1P-mediated phosphorylation of IKK but not phosphorylation of STAT3 (Tyr705) (Fig. 6C), supporting the notion that Bcl10 is involved in the signaling pathway leading to IKK phosphorylation and subsequently to NF-κB activation.
Akt activation is crucial for S1P-induced NF-κB activation.
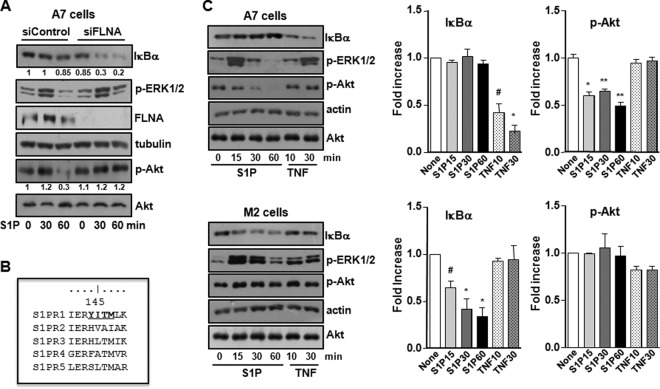
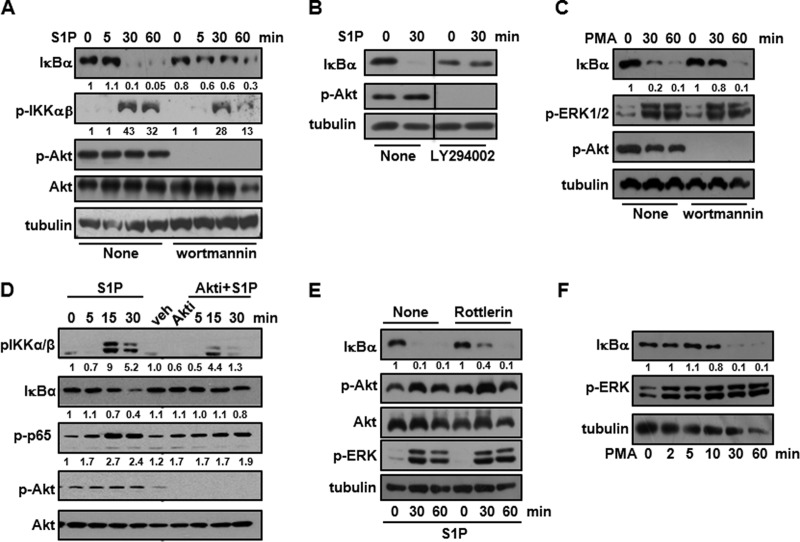
Our data suggest that the activation of NF-κB by exogenous S1P inversely correlates with the presence of FLNA. To convincingly demonstrate that FLNA negatively regulates S1P-mediated NF-κB activation, FLNA-expressing A7 cells were transfected with siRNA targeting FLNA. Western blot analysis shows that residual FLNA expression after silencing was 19% ± 0.9% (n = 6). Decreasing FLNA expression restored the ability of S1P to induce IκBα degradation (Fig. 7A). It was then important to determine how FLNA negatively regulates S1P-mediated signaling leading to NF-κB activation. It was recently shown that FLNA can function as a negative regulator of the PI3K/Akt signaling of some GPCRs containing both a FLNA binding motif and the PI3K regulatory p85 subunit binding YXXM motif in close proximity in an intracellular loop sequence (24). As a consequence, upon ligand treatment, the PI3K regulatory p85 subunit dissociates from the GPCR, which now associates with FLNA. This switch causes an inhibition of PI3K/Akt signaling (24). Interestingly, S1PR1 is the sole S1P receptor that contains both of these motifs (24) (Fig. 7B). Thus, it was important to examine the effect of FLNA expression on Akt activation induced by S1P. Although, as expected, S1P stimulated the phosphorylation of ERK1/2 in both M2 and A7 cells, it did not have any effect on the high basal phospho-Akt levels in M2 cells. However, remarkably, S1P diminished Akt phosphorylation in FLNA-expressing A7 cells in a time-dependent manner (Fig. 7C), whereas after downregulation of FLNA, S1P no longer inhibited the phosphorylation of Akt (Fig. 7A). These results suggest that in the presence of FLNA, S1P negatively regulates the PI3K/Akt pathway, while the absence of FLNA enables high basal PI3K/Akt activation, which in turn is critical for IKK phosphorylation and NF-κB activation. To substantiate the role of PI3K/Akt in S1P-induced NF-κB activation, M2 cells were treated with the PI3K inhibitors wortmannin and LY294002 (Fig. 8A and B). Wortmannin treatment reduced IKK phosphorylation, and both wortmannin and LY294002 delayed S1P-induced IκBα degradation (Fig. 8A and B). Similarly, IκBα degradation induced by PMA was also partially suppressed by wortmannin treatment, whereas PMA-induced ERK1/2 phosphorylation was unaltered (Fig. 8C). Moreover, MK-2206, a potent allosteric Akt inhibitor, also significantly reduced IKK phosphorylation and degradation of IκBα as well as phosphorylation of p65, concomitantly with the inhibition of Akt phosphorylation (Fig. 8D). We also investigated the relationship between PKCδ and Akt in S1P-induced NF-κB activation. While specific pharmacological inhibition of PKCδ by rottlerin treatment (25) slightly delayed IκBα degradation, it had no discernible effect on the phosphorylation of Akt (Fig. 8E). Moreover, in contrast to the inability of S1P to stimulate NF-κB in FLNA-expressing A7 cells, direct activation of PKC with PMA induced the degradation of IκBα in these cells (Fig. 8F).
FIG 7.
S1P decreases Akt phosphorylation in FLNA-expressing cells. (A) A7 cells were transfected with control siRNA or siFLNA. After overnight starvation, cells were treated with S1P (100 nM) for the indicated times. (B) Sequence alignment of the S1PRs showing that only S1PR1 contains both the FLNA binding motif and the YXXM sequence in close proximity in intracellular loop 2. (C) A7 and M2 cells were serum starved overnight and stimulated with S1P (100 nM) or TNF (10 ng/ml), as indicated. Cell lysates were analyzed by immunoblotting with the indicated antibodies. Tubulin, actin, and total Akt were used as indicated to ensure equal loading and transfer. Histograms show densitometric quantification of IκBα and p-Akt for A7 and M2 cells. #, P < 0.05; *, P < 0.01; **, P < 0.001 (compared to untreated cells [None]). Blots are representative of results from at least three independent experiments.
FIG 8.
Role of Akt activation in S1P-induced NF-κB activation. (A to E) M2 cells were starved overnight; preincubated for 30 min with 100 nM wortmannin (A and C), 50 μM LY294002 (B),100 nM Mk2206 (D), or 1 μM rottlerin (E); and then stimulated with S1P (100 nM) or PMA (100 nM) for the indicated times. (F) A7 cells were starved overnight and treated with PMA (100 nM), as indicated. Cell lysate proteins were analyzed by immunoblotting with the indicated antibodies. Tubulin or Akt was used to ensure equal loading and transfer. Numbers indicate relative protein levels determined by densitometry. Blots are representative of results from two or three independent experiments.
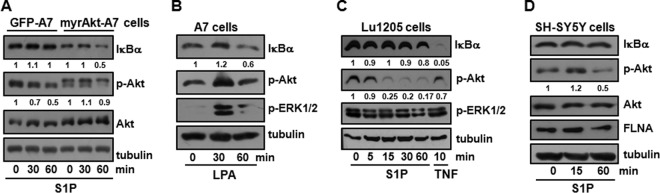
To further support the role of activated Akt in S1P-induced NF-κB activation, we generated A7 cells expressing constitutively active, myristoylated Akt (26). Remarkably, in these cells, S1P was then able to induce IκBα degradation even in the presence of FLNA (Fig. 9A), in sharp contrast to control transfected cells. Interestingly, LPA, another bioactive lysolipid that is a ligand for a closely related family of GPCRs (5), unlike S1P, was able to induce IκBα degradation even in A7 cells (Fig. 9B).
FIG 9.
Role of Akt in S1P-induced IκBα degradation in FLNA-expressing cells. (A) A7 cells expressing GFP or myristoylated Akt (myrAkt) were starved overnight and stimulated with S1P (100 nM) for the indicated times. Numbers indicate relative protein levels determined by densitometry, normalized to the levels in the respective untreated cells. (B) A7 cells were serum starved overnight and treated with LPA (1 μM). (C and D) Lu1205 cells (C) and SH-SY5Y cells (D) were serum starved overnight and stimulated with S1P (100 nM) or TNF (10 ng/ml), as indicated. In panels A to D, cell lysates were analyzed by immunoblotting with the indicated antibodies. Where indicated, tubulin and total Akt were used to ensure equal loading and transfer. Numbers indicate relative protein levels determined by densitometry.
Next, we wondered whether S1P also reduced Akt activation and was unable to activate NF-κB in other FLNA-expressing cells. Similarly to A7 cells, in Lu1205 melanoma cells that also express FLNA (Fig. 1C), S1P, in contrast to TNF, was unable to induce IκBα degradation (Fig. 9C). Importantly, as with A7 cells, S1P also greatly reduced the phosphorylation of Akt in Lu1205 cells, whereas treatment with TNF had no significant effect (Fig. 9C). Because Lu1205 cells have a BRAFV600E mutation resulting in the activation of ERK1/2, levels of phosphorylated ERK1/2 (p-ERK1/2) are high and do not change with S1P treatment. Moreover, in SH-SY5Y neuroblastoma cells that also express FLNA, S1P did not induce IκBα degradation, while it reduced the levels of phosphorylated Akt (Fig. 9D). Altogether, these results indicate that in the presence of FLNA, S1P reduces the phosphorylation of Akt and can no longer stimulate NF-κB.
DISCUSSION
We previously showed that intracellular S1P generated by SphK1 functions as a cofactor for TRAF2 and is required to link TNF signaling to NF-κB activation in melanoma cells (13). Interestingly, activation of NF-κB by TNF occurs only in the presence of FLNA (11), which interacts with many signaling proteins, including TRAF2 (10, 11). As SphK1 also physically interacts with FLNA (9), FLNA may act as a scaffold to allow localized production of S1P, which in turn binds and activates TRAF2, leading to NF-κB signaling. Recent studies have shown that TRAF-interacting protein (TRIP), a known cellular binding partner of TRAF2 (27), inhibits TNF-induced NF-κB activation by inhibiting the binding of S1P to TRAF2 and thus suppressing its E3 ubiquitin ligase activity (28).
We have now demonstrated that FLNA negatively regulates the activation of NF-κB induced by extracellular S1P in melanoma cells. Thus, S1P activates NF-κB only in M2 cells that are devoid of FLNA. In contrast, in their counterpart A7 melanoma cells that express FLNA, S1P does not activate NF-κB unless the expression of FLNA is downregulated. Several previous reports suggested that S1P can activate NF-κB via S1PRs (14, 15, 29–34). Although in some studies, the S1PR involved was not identified and the concentration of S1P used was too high to substantiate the involvement of S1PR-mediated events (35), others have clearly implicated S1PR1 to -3 in different cell types. For example, S1P-induced activation of NF-κB in human umbilical vein endothelial cells (HUVECs) was abrogated by pertussis toxin, suggesting the involvement of inhibitory G protein (Gi)-dependent mechanisms (32). However, S1P induced the activation of NF-κB in HEK293 cells overexpressing S1PR2 or S1PR3 but not S1PR1 (33). Subsequently, specific antisense oligonucleotides were used to show that the activation of NF-κB in HUVECs requires mainly S1PR3 and, to a much lesser extent, S1PR1 (15). Moreover, pharmacological and siRNA experiments substantiated the involvement of S1PR1 and S1PR3 in NF-κB activation in cooperation with lipopolysaccharide (LPS) to induce inflammatory molecules and leukocyte adhesion in endothelial cells (29). Surprisingly, S1PRs have been linked not only to stimulatory pathways leading to the activation of NF-κB but also to inhibitory pathways in HUVECs. Whereas the stimulatory pathway was mediated mainly by S1PR3 coupled to G12/13 leading to NF-κB activation, the inhibitory pathway was mediated by S1PR1 coupled to Gi and PI3K/Akt leading to the activation of endothelial nitric oxide synthase (eNOS) and the production of NO, which suppressed TNF-mediated NF-κB activation (31). However, in HeLa cells, S1PR2, but not S1PR1 or S1PR3, was responsible for the activation of NF-κB induced by extracellular S1P (14). In addition, S1PR2 induced endothelial inflammation by the activation of the Rho-ROCK (Rho-associated protein kinase) pathway leading to NF-κB activation (34). Using M2 melanoma cells, we have now provided evidence showing that both S1PR1 and S1PR2 are involved in the activation of NF-κB by S1P. This is based on the use of S1PR1- and S1PR2-specific antagonists and agonists as well as the downregulation of these receptors. Of note, siRNA targeted to S1PR2 unexpectedly reduced the expression levels of both S1PR2 and S1PR1 and markedly suppressed the activation of NF-κB by exogenous S1P. Activation of NF-κB by S1P in FLNA-deficient melanoma cells might contribute to the increased expression of proinflammatory cytokines and chemokines, which have been associated with tumor progression (36), and increase the survival of cancer cells, which directly enhances tumor growth.
Although many PKC isoforms, including atypical PKCs (37), have been implicated in NF-κB activation, we noted that S1P induced time-dependent phosphorylation of PKCδ without affecting the activation of other PKCs. Moreover, downregulation of PKCδ greatly reduced S1P-mediated IKK phosphorylation and NF-κB activation, supporting a role for this PKC isoform in NF-κB activation. Similarly, in response to LPA, PKCδ was required for NF-κB activation and IL-8 secretion in bronchial epithelial cells (20). In this regard, PMA-induced NF-κB activation was shown to involve PKCδ and PKCε (38). Moreover, PKCδ orchestrates p65 activation in response to TNF (39, 40).
It has been suggested that in response to LPA, PKC-dependent phosphorylation of the scaffold protein CARD (caspase recruitment domain) and membrane-associated guanylate kinase-like domain-containing protein 3 (CARMA3) (41) induces a conformational change and permits its interaction with Bcl10 and MALT1 (mucosa-associated lymphoid tissue lymphoma translocation protein 1), and oligomerized MALT1 interacts with the ubiquitin ligase TRAF6. The subsequent ubiquitination of the IKK complex then results in optimal IKK-mediated NF-κB activation (23, 42). Likewise, we found that Bcl10 is involved in S1P-induced IKK phosphorylation and NF-κB activation, and its downregulation greatly reduced this pathway. Hence, it is likely that a similar signaling pathway involving PKCδ and the CARMA3-Bcl10-MALT1 complex that activates IKK in LPA signaling also exists downstream of S1P signaling.
Activation of NF-κB by the S1P/S1PR axis was not observed in several types of FLNA-expressing melanoma cells. This raised an intriguing question: how does FLNA negatively regulate this pathway? Our results support the proposal that in FLNA-expressing cells, for GPCRs that contain a FLNA binding motif and the PI3K regulatory p85 subunit binding motif in close proximity, upon ligand binding (such as S1P), the cognate receptor switches its binding preference from p85 to FLNA (24). This switch results in the dissociation of p85 and inhibition of the PI3K/Akt pathway. Although FLNA expression does not regulate the activation of NF-κB induced by LPA, it was previously shown that FLNA interacts with other GPCRs, such as the mu opioid receptor and the somatostatin sst2 receptor, to negatively regulate the PI3K-Akt pathway (24). However, although FLNA expression was originally implicated in cell migration, invasion, and metastasis, recent reports suggest that under certain conditions, it prevents tumor formation or progression (10). In this regard, it is tempting to speculate that inhibition of the PI3K/Akt pathway by FLNA might contribute to these effects.
Several lines of evidence support the notion that activation of Akt is necessary for the full activation of the NF-κB pathway by S1P. First, inhibitors of PI3K or Akt greatly reduced S1P-induced IKK activation. Second, S1P reduced Akt phosphorylation only in FLNA-expressing cells. Third, downregulation of FLNA restored the ability of S1P to induce IκBα degradation and prevented it from decreasing the phosphorylation of Akt. Finally, overexpression of myristoylated, constitutively active Akt allowed S1P to induce IκBα degradation in A7 FLNA-expressing cells. This is consistent with data from previous studies showing that Akt-mediated regulation of NF-κB is dependent on its phosphorylation of IKKα, a prerequisite for the phosphorylation of p65 by IKKα and -β (43). Intriguingly, although both PKCδ and PI3K/Akt are important for S1P-induced NF-κB activation in FLNA-deficient M2 cells, our data suggest that FLNA expression suppresses S1P-mediated NF-κB activation only by modulation of the PI3K/Akt pathway. Taken together, our results support a negative role for FLNA in S1P-mediated NF-κB activation in melanoma cells by inhibition of Akt.
ACKNOWLEDGMENTS
We thank Y. Ohta and T. Stossel (Brigham and Women's Hospital) for the M2 and A7 cell lines and P. B. Fisher (VCU, Richmond, VA) for WM35 and FM516 cells. SEW2871 was generously provided by Romina Gamberale and Mercedes Borge (CONICET, Argentina). Rottlerin was a gift of Alejandro Urtreger (CONICET, Argentina). Akt antibody was provided by Diego Crocci (CONICET, Argentina). S1PR1, S1PR2, and S1PR3 antibodies were a generous gift of Silvia Belmonte (CONICET, Argentina).
REFERENCES
- 1.Maceyka M, Spiegel S. 2014. Sphingolipid metabolites in inflammatory disease. Nature 510:58–67. doi: 10.1038/nature13475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pyne NJ, Pyne S. 2010. Sphingosine 1-phosphate and cancer. Nat Rev Cancer 10:489–503. doi: 10.1038/nrc2875. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel S, Milstien S. 2011. The outs and the ins of sphingosine-1-phosphate in immunity. Nat Rev Immunol 11:403–415. doi: 10.1038/nri2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alvarez SE, Milstien S, Spiegel S. 2007. Autocrine and paracrine roles of sphingosine-1-phosphate. Trends Endocrinol Metab 18:300–307. doi: 10.1016/j.tem.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 5.Kihara Y, Maceyka M, Spiegel S, Chun J. 2014. Lysophospholipid receptor nomenclature review: IUPHAR review 8. Br J Pharmacol 171:3575–3594. doi: 10.1111/bph.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maceyka M, Harikumar KB, Milstien S, Spiegel S. 2012. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol 22:50–60. doi: 10.1016/j.tcb.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nijnik A, Clare S, Hale C, Chen J, Raisen C, Mottram L, Lucas M, Estabel J, Ryder E, Adissu H, Sanger Mouse Genetics Project, Adams NC, Ramirez-Solis R, White JK, Steel KP, Dougan G, Hancock RE. 2012. The role of sphingosine-1-phosphate transporter Spns2 in immune system function. J Immunol 189:102–111. doi: 10.4049/jimmunol.1200282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mitra P, Oskeritzian CA, Payne SG, Beaven MA, Milstien S, Spiegel S. 2006. Role of ABCC1 in export of sphingosine-1-phosphate from mast cells. Proc Natl Acad Sci U S A 103:16394–16399. doi: 10.1073/pnas.0603734103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maceyka M, Alvarez SE, Milstien S, Spiegel S. 2008. Filamin A links sphingosine kinase 1 and sphingosine-1-phosphate receptor 1 at lamellipodia to orchestrate cell migration. Mol Cell Biol 28:5687–5697. doi: 10.1128/MCB.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Savoy RM, Ghosh PM. 2013. The dual role of filamin A in cancer: can't live with (too much of) it, can't live without it. Endocr Relat Cancer 20:R341–R356. doi: 10.1530/ERC-13-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leonardi A, Ellinger-Ziegelbauer H, Franzoso G, Brown K, Siebenlist U. 2000. Physical and functional interaction of filamin (actin-binding protein-280) and tumor necrosis factor receptor-associated factor 2. J Biol Chem 275:271–278. doi: 10.1074/jbc.275.1.271. [DOI] [PubMed] [Google Scholar]
- 12.Xia P, Wang L, Moretti PA, Albanese N, Chai F, Pitson SM, D'Andrea RJ, Gamble JR, Vadas MA. 2002. Sphingosine kinase interacts with TRAF2 and dissects tumor necrosis factor-alpha signaling. J Biol Chem 277:7996–8003. doi: 10.1074/jbc.M111423200. [DOI] [PubMed] [Google Scholar]
- 13.Alvarez SE, Harikumar KB, Hait NC, Allegood J, Strub GM, Kim EY, Maceyka M, Jiang H, Luo C, Kordula T, Milstien S, Spiegel S. 2010. Sphingosine-1-phosphate is a missing cofactor for the E3 ubiquitin ligase TRAF2. Nature 465:1084–1088. doi: 10.1038/nature09128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blom T, Bergelin N, Meinander A, Lof C, Slotte JP, Eriksson JE, Tornquist K. 2010. An autocrine sphingosine-1-phosphate signaling loop enhances NF-kappaB-activation and survival. BMC Cell Biol 11:45. doi: 10.1186/1471-2121-11-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura T, Tomura H, Mogi C, Kuwabara A, Ishiwara M, Shibasawa K, Sato K, Ohwada S, Im DS, Kurose H, Ishizuka T, Murakami M, Okajima F. 2006. Sphingosine 1-phosphate receptors mediate stimulatory and inhibitory signalings for expression of adhesion molecules in endothelial cells. Cell Signal 18:841–850. doi: 10.1016/j.cellsig.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 16.O'Sullivan MJ, Hirota N, Martin JG. 2014. Sphingosine 1-phosphate (S1P) induced interleukin-8 (IL-8) release is mediated by S1P receptor 2 and nuclear factor kappaB in BEAS-2B cells. PLoS One 9:e95566. doi: 10.1371/journal.pone.0095566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunningham CC, Gorlin JB, Kwiatkowski DJ, Hartwig JH, Janmey PA, Byers HR, Stossel TP. 1992. Actin-binding protein requirement for cortical stability and efficient locomotion. Science 255:325–327. doi: 10.1126/science.1549777. [DOI] [PubMed] [Google Scholar]
- 18.Mattioli I, Sebald A, Bucher C, Charles RP, Nakano H, Doi T, Kracht M, Schmitz ML. 2004. Transient and selective NF-kappa B p65 serine 536 phosphorylation induced by T cell costimulation is mediated by I kappa B kinase beta and controls the kinetics of p65 nuclear import. J Immunol 172:6336-6344. doi: 10.4049/jimmunol.172.10.6336. [DOI] [PubMed] [Google Scholar]
- 19.Davis MD, Clemens JJ, Macdonald TL, Lynch KR. 2005. Sphingosine 1-phosphate analogs as receptor antagonists. J Biol Chem 280:9833–9841. doi: 10.1074/jbc.M412356200. [DOI] [PubMed] [Google Scholar]
- 20.Cummings R, Zhao Y, Jacoby D, Spannhake EW, Ohba M, Garcia JG, Watkins T, He D, Saatian B, Natarajan V. 2004. Protein kinase Cdelta mediates lysophosphatidic acid-induced NF-kappaB activation and interleukin-8 secretion in human bronchial epithelial cells. J Biol Chem 279:41085–41094. doi: 10.1074/jbc.M404045200. [DOI] [PubMed] [Google Scholar]
- 21.Wang D, You Y, Lin PC, Xue L, Morris SW, Zeng H, Wen R, Lin X. 2007. Bcl10 plays a critical role in NF-kappaB activation induced by G protein-coupled receptors. Proc Natl Acad Sci U S A 104:145–150. doi: 10.1073/pnas.0601894104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Markou T, Yong CS, Sugden PH, Clerk A. 2006. Regulation of protein kinase C delta by phorbol ester, endothelin-1, and platelet-derived growth factor in cardiac myocytes. J Biol Chem 281:8321–8331. doi: 10.1074/jbc.M508398200. [DOI] [PubMed] [Google Scholar]
- 23.Klemm S, Zimmermann S, Peschel C, Mak TW, Ruland J. 2007. Bcl10 and Malt1 control lysophosphatidic acid-induced NF-kappaB activation and cytokine production. Proc Natl Acad Sci U S A 104:134–138. doi: 10.1073/pnas.0608388103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Najib S, Saint-Laurent N, Esteve JP, Schulz S, Boutet-Robinet E, Fourmy D, Lattig J, Mollereau C, Pyronnet S, Susini C, Bousquet C. 2012. A switch of G protein-coupled receptor binding preference from phosphoinositide 3-kinase (PI3K)-p85 to filamin A negatively controls the PI3K pathway. Mol Cell Biol 32:1004–1016. doi: 10.1128/MCB.06252-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berardi DE, Flumian C, Rodriguez CE, Diaz Bessone MI, Cirigliano SM, Bal de Kier Joffe ED, Fiszman GL, Urtreger AJ, Todaro LB. 3 September 2015. PKCdelta inhibition impairs mammary cancer proliferative capacity but selects cancer stem cells, involving autophagy. J Cell Biochem doi: 10.1002/jcb.25358. [DOI] [PubMed] [Google Scholar]
- 26.Kohn AD, Summers SA, Birnbaum MJ, Roth RA. 1996. Expression of a constitutively active Akt Ser/Thr kinase in 3T3-L1 adipocytes stimulates glucose uptake and glucose transporter 4 translocation. J Biol Chem 271:31372–31378. doi: 10.1074/jbc.271.49.31372. [DOI] [PubMed] [Google Scholar]
- 27.Lee SY, Lee SY, Choi Y. 1997. TRAF-interacting protein (TRIP): a novel component of the tumor necrosis factor receptor (TNFR)- and CD30-TRAF signaling complexes that inhibits TRAF2-mediated NF-kappaB activation. J Exp Med 185:1275–1285. doi: 10.1084/jem.185.7.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park ES, Choi S, Shin B, Yu J, Yu J, Hwang JM, Yun H, Chung YH, Choi JS, Choi Y, Rho J. 2015. Tumor necrosis factor (TNF) receptor-associated factor (TRAF)-interacting protein (TRIP) negatively regulates the TRAF2 ubiquitin-dependent pathway by suppressing the TRAF2-sphingosine 1-phosphate (S1P) interaction. J Biol Chem 290:9660–9673. doi: 10.1074/jbc.M114.609685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fernandez-Pisonero I, Duenas AI, Barreiro O, Montero O, Sanchez-Madrid F, Garcia-Rodriguez C. 2012. Lipopolysaccharide and sphingosine-1-phosphate cooperate to induce inflammatory molecules and leukocyte adhesion in endothelial cells. J Immunol 189:5402–5410. doi: 10.4049/jimmunol.1201309. [DOI] [PubMed] [Google Scholar]
- 30.Hsieh HL, Wu CB, Sun CC, Liao CH, Lau YT, Yang CM. 2006. Sphingosine-1-phosphate induces COX-2 expression via PI3K/Akt and p42/p44 MAPK pathways in rat vascular smooth muscle cells. J Cell Physiol 207:757–766. doi: 10.1002/jcp.20621. [DOI] [PubMed] [Google Scholar]
- 31.Kimura T, Tomura H, Mogi C, Kuwabara A, Damirin A, Ishizuka T, Sekiguchi A, Ishiwara M, Im DS, Sato K, Murakami M, Okajima F. 2006. Role of scavenger receptor class B type I and sphingosine 1-phosphate receptors in high density lipoprotein-induced inhibition of adhesion molecule expression in endothelial cells. J Biol Chem 281:37457–37467. doi: 10.1074/jbc.M605823200. [DOI] [PubMed] [Google Scholar]
- 32.Lee H, Lin CI, Liao JJ, Lee YW, Yang HY, Lee CY, Hsu HY, Wu HL. 2004. Lysophospholipids increase ICAM-1 expression in HUVEC through a Gi- and NF-kappaB-dependent mechanism. Am J Physiol Cell Physiol 287:C1657–C1666. doi: 10.1152/ajpcell.00172.2004. [DOI] [PubMed] [Google Scholar]
- 33.Siehler S, Wang Y, Fan X, Windh RT, Manning DR. 2001. Sphingosine 1-phosphate activates nuclear factor-kappa B through Edg receptors. Activation through Edg-3 and Edg-5, but not Edg-1, in human embryonic kidney 293 cells. J Biol Chem 276:48733–48739. [DOI] [PubMed] [Google Scholar]
- 34.Zhang G, Yang L, Kim GS, Ryan K, Lu S, O'Donnell RK, Spokes K, Shapiro N, Aird WC, Kluk MJ, Yano K, Sanchez T. 2013. Critical role of sphingosine-1-phosphate receptor 2 (S1PR2) in acute vascular inflammation. Blood 122:443–455. doi: 10.1182/blood-2012-11-467191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hsieh HL, Sun CC, Wu CB, Wu CY, Tung WH, Wang HH, Yang CM. 2008. Sphingosine 1-phosphate induces EGFR expression via Akt/NF-kappaB and ERK/AP-1 pathways in rat vascular smooth muscle cells. J Cell Biochem 103:1732–1746. doi: 10.1002/jcb.21563. [DOI] [PubMed] [Google Scholar]
- 36.Lin WW, Karin M. 2007. A cytokine-mediated link between innate immunity, inflammation, and cancer. J Clin Invest 117:1175–1183. doi: 10.1172/JCI31537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Diaz-Meco MT, Moscat J. 2012. The atypical PKCs in inflammation: NF-kappaB and beyond. Immunol Rev 246:154–167. doi: 10.1111/j.1600-065X.2012.01093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Holden NS, Squires PE, Kaur M, Bland R, Jones CE, Newton R. 2008. Phorbol ester-stimulated NF-kappaB-dependent transcription: roles for isoforms of novel protein kinase C. Cell Signal 20:1338–1348. doi: 10.1016/j.cellsig.2008.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Garg R, Caino MC, Kazanietz MG. 2013. Regulation of transcriptional networks by PKC isozymes: identification of c-Rel as a key transcription factor for PKC-regulated genes. PLoS One 8:e67319. doi: 10.1371/journal.pone.0067319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lu ZG, Liu H, Yamaguchi T, Miki Y, Yoshida K. 2009. Protein kinase Cdelta activates RelA/p65 and nuclear factor-kappaB signaling in response to tumor necrosis factor-alpha. Cancer Res 69:5927–5935. doi: 10.1158/0008-5472.CAN-08-4786. [DOI] [PubMed] [Google Scholar]
- 41.Blonska M, Lin X. 2011. NF-kappaB signaling pathways regulated by CARMA family of scaffold proteins. Cell Res 21:55–70. doi: 10.1038/cr.2010.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sun W, Yang J. 2010. Molecular basis of lysophosphatidic acid-induced NF-kappaB activation. Cell Signal 22:1799–1803. doi: 10.1016/j.cellsig.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bai D, Ueno L, Vogt PK. 2009. Akt-mediated regulation of NFkappaB and the essentialness of NFkappaB for the oncogenicity of PI3K and Akt. Int J Cancer 125:2863–2870. doi: 10.1002/ijc.24748. [DOI] [PMC free article] [PubMed] [Google Scholar]