Figure 2.
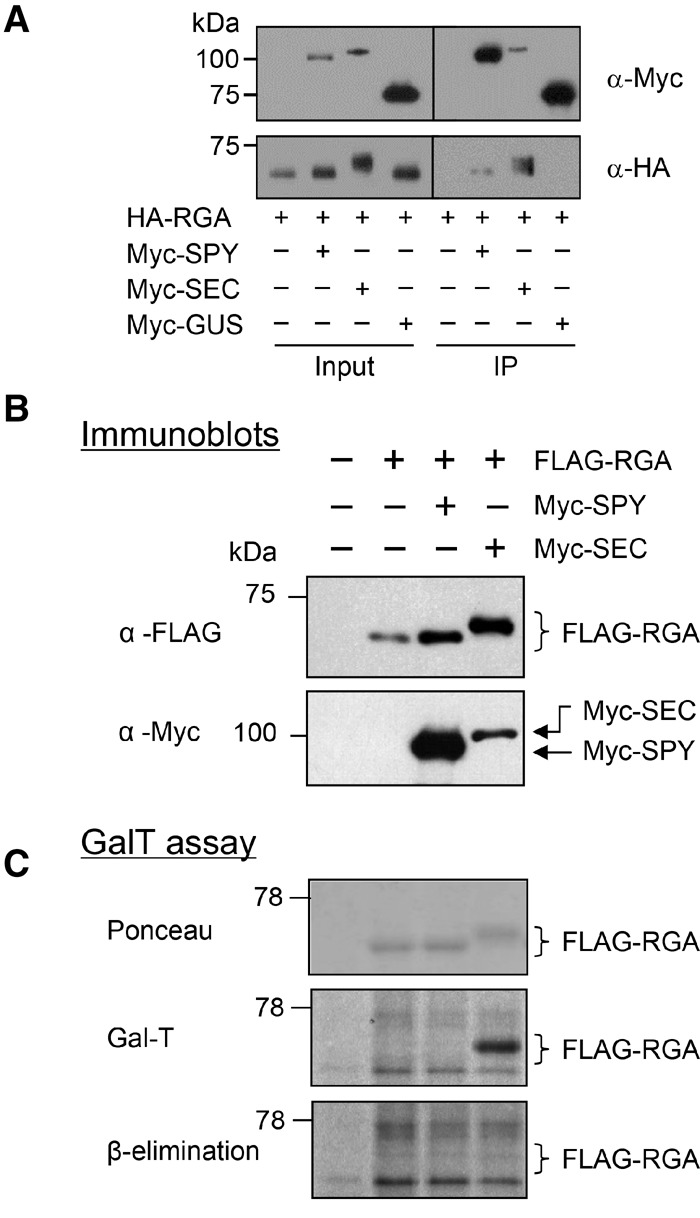
Both SEC and SPY bind to RGA, but only SEC O-GlcNAcylates RGA in the tobacco transient expression system. (A) Co-IP of RGA with SEC and SPY in planta. HA-RGA was transiently expressed alone or coexpressed with Myc-SPY, Myc-SEC, or Myc-GUS-NLS in N. benthamiana. Total protein extracts were immunoprecipitated with anti-Myc agarose beads; input and immunoprecipitation (IP) samples were analyzed by immunoblotting using anti-HA and anti-Myc antibodies separately. (B,C) RGA is O-GlcNAcylated by SEC but not by SPY in the tobacco transient expression system. Flag-RGA was expressed alone or coexpressed with Myc-SPY or Myc-SEC in N. benthamiana (as labeled above the blot in B). (B) Immunoblot analysis using total protein extracts and anti-Flag or anti-Myc antibodies; coexpression of RGA and SEC causes a detectable mobility shift of RGA. (C) A GalT assay followed by β elimination confirmed that the mobility-shifted RGA in the RGA + SEC sample is caused by O-GlcNAcylation. The blot contains equal amounts of affinity-purified Flag-RGA proteins, as indicated by the Ponceau-S-stained blot image. GalT labeled both N- and O-linked GlcNAcylated proteins with [3H]-galactose. The Flag-RGA signal (in the RGA + SEC sample) disappeared after β elimination. The remaining signals on the blot are N-GlcNAcylated proteins. Brackets indicate the position of unmodified and modified RGA. In A–C, 6% SDS-PAGE long (12-cm) gels were used to show differential electrophoretic mobility of GlcNAcylated RGA versus unmodified RGA proteins.
