FIG 3.
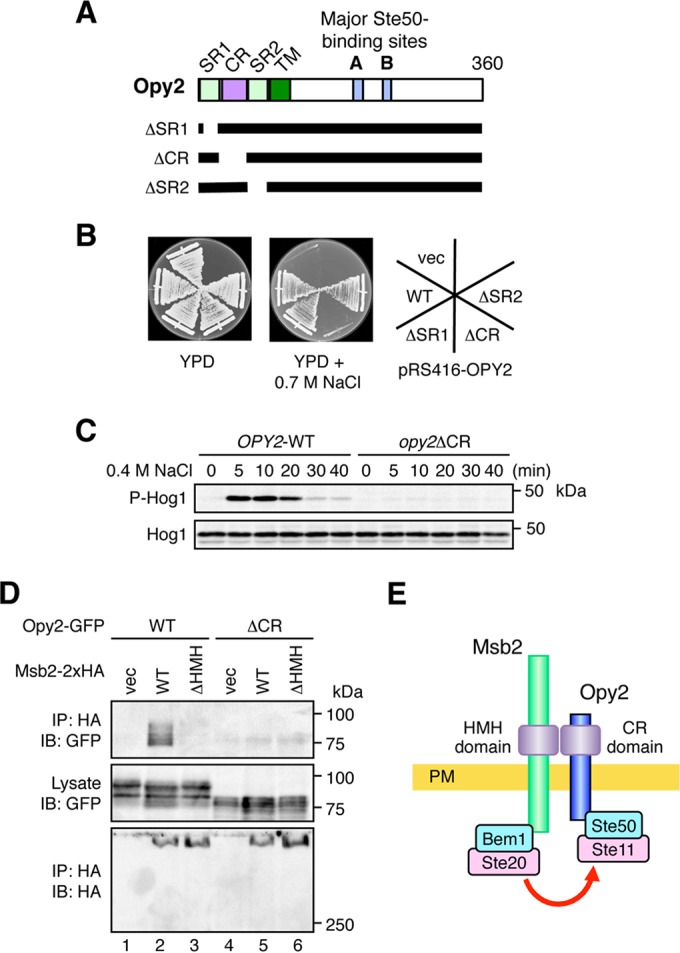
The Opy2 CR domain binds to the Msb2 HMH domain. (A) Schematic model of Opy2. The horizontal black bars represent the structures of the Opy2 deletion mutants. SR1 and SR2, Ser-rich domains 1 and 2, respectively; CR, Cys-rich domain; TM, transmembrane domain. (B) Osmosensitivity of opy2 mutants. The yeast strain KY477 (ssk2Δ ssk22Δ opy2Δ) was transformed with a single-copy plasmid carrying either the wild-type (WT) OPY2 or one of the indicated opy2 deletion constructs. Transformed cells were streaked on yeast extract-peptone-dextrose (YPD) plates in the absence or presence of an osmostressor (0.7 M NaCl). The plates were photographed after 3 days of incubation at 30°C. vec, vector control. (C) Hog1 phosphorylation in response to osmotic stress. KY477 was transformed with either the OPY2 wild-type or opy2ΔCR strain. Cells were collected at the indicated times after addition of 0.4 M NaCl, and the amounts of phosphorylated Hog1 (P-Hog1) and total Hog1 (Hog1) were examined by immunoblotting of the total cell lysate. (D) The Opy2 CR domain is necessary for Msb2-Opy2 interaction. TM257 was cotransformed with expression plasmids for the Opy2-GFP wild type or Opy2ΔCR-GFP (ΔCR), both of which were under the control of the GAL1 promoter, together with Msb2-2×HA (wild type), Msb2-ΔHMH-2×HA, or the vector control. Cell growth, preparation of cell lysates, immunoprecipitation, and immunoblotting were as described in the legend to Fig. 2A. Buffer A containing 1.0% digitonin was used. (E) Schematic model of the Msb2-Opy2 interaction. PM, plasma membrane.
