FIG 4.
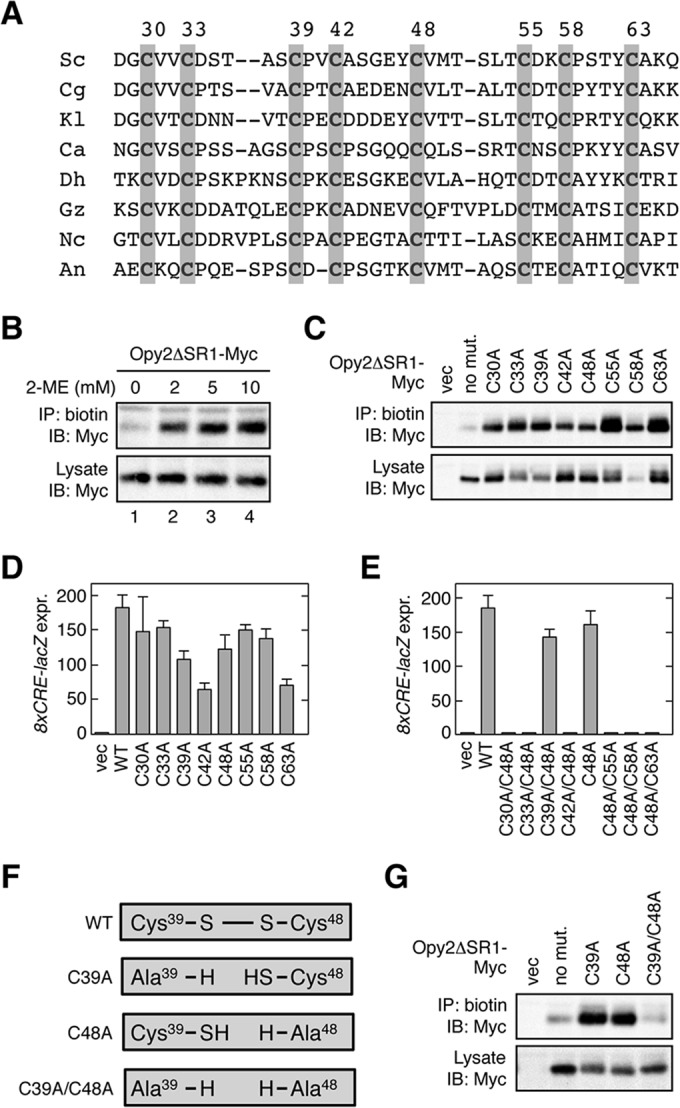
Conserved cysteine residues in the Opy2 CR domain. (A) Comparison of the amino acid sequences of the Opy2 CR domain from various yeast and fungal species. Gray shading highlights conserved cysteine residues. Numbers are amino acid positions in the S. cerevisiae Opy2. Sc, Saccharomyces cerevisiae; Cg, Candida glabrata; Kl, Kluyveromyces lactis; Ca, Candida albicans; Dh, Debaryomyces hansenii; Gz, Gibberella zeae; Nc, Neurospora crassa; An, Aspergillus nidulans. (B) Biotin labeling of reduced cysteine residues in Opy2 using maleimide-PEG2-biotin. KY477 was transformed with a plasmid that expresses Opy2ΔSR1-Myc under the control of the GAL1 promoter. Expression was induced by 2% galactose for 2 h. A membrane fraction was prepared and was incubated with the indicated concentration of 2-ME at 37°C for 20 min. After removal of 2-ME by two rounds of washing, samples were incubated with 0.2 mM maleimide-PEG2-biotin at 30°C for 5 min. The membrane was then solubilized with 1.0% Triton X-100 plus 0.2% SDS. Biotinylated Opy2ΔSR1-Myc was immunoprecipitated using streptavidin-agarose and was detected using an anti-Myc antibody. (C) Biotin labeling of Opy2 Cys-to-Ala mutants using maleimide-PEG2-biotin. KY477 was transformed with the indicated mutants of Opy2ΔSR1-Myc or an empty vector (vec). Expression was induced by 2% galactose for 2 h. A membrane fraction was prepared and incubated with 0.2 mM maleimide-PEG2-biotin at 30°C for 5 min. Detection of biotinylation was as described in the legend to panel B. (D and E) Osmostress-induced expression of the Hog1-specific reporter gene 8×CRE-lacZ. KY477 was cotransformed with the reporter plasmid pRS416-8×CRE-lacZ and a single-copy plasmid carrying the wild-type (WT) OPY2 gene (pRS414-OPY2) or the indicated mutants. Cells were grown in CAD medium and were stimulated with 0.4 M NaCl for 30 min. The activity of β-galactosidase in cell extracts was normalized to the cell density and is expressed as Miller units (35). All reporter assays were carried out in triplicate (or more times) with independent cultures. Error bars represent SDs. expr., expression. (F) Effects of Cys-to-Ala mutations on a disulfide bond. If Cys39 and Cys48 form a disulfide bond in the wild-type Opy2 molecule, either the C39A or the C48A mutation generates a reactive cysteine residue. In contrast, the C39A/C48A double mutant should have no reactive cysteine. (G) Biotin labeling of Opy2 Cys-to-Ala mutants using maleimide-PEG2-biotin. Expression of Opy2ΔSR1-Myc mutants, labeling of cysteines with 0.2 mM maleimide-PEG2-biotin, and detection of biotinylation were conducted as described in the legend to panel B.
