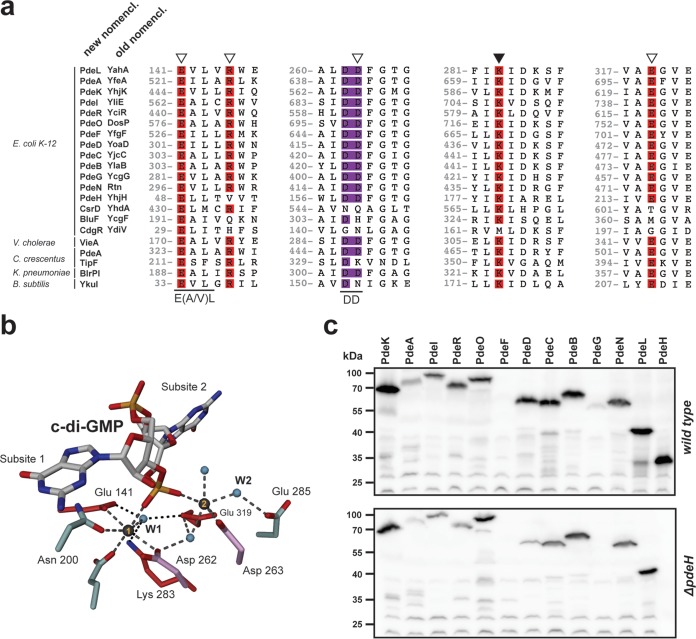
FIG 1.
Conservation of c-di-GMP-specific phosphodiesterases. (a) ClustalW alignment of PdeL with endogenous and exogenous phosphodiesterases and noncatalytic EAL domains. Regions containing residues involved in substrate binding (open triangles) and catalysis (closed triangle) are highlighted. Amino acid numbering refers to the numbering for PdeL. The double-aspartic-acid motif (DD) is displayed in purple, in analogy to panel b. (b) Atomic organization of the catalytic site of a PdeL monomer. The two essential metals are displayed as gray spheres. Conserved residues involved in metal coordination or catalysis are displayed in red. The double-aspartic-acid motif (Asp262 and Asp263; shown in purple) is involved in metal ion coordination as well as coordination of the catalytic water (small blue spheres). The same is true for the glutamic acid at position E141, which is part of the conserved E(A/V)L motif. (c) Immunoblot analysis of PDEs in E. coli wild-type and ΔpdeH mutant strains. PDEs were tagged at their C termini with a 3×Flag tag and were analyzed with an anti-Flag antibody. Cells were grown in tryptone broth (TB) at 37°C and harvested at an OD600 of 0.8. Note that in both strain backgrounds, all pde genes were expressed, with the exception of pdeF. PdeG levels were low in the wild type, and the protein seemed to be absent in the ΔpdeH mutant.