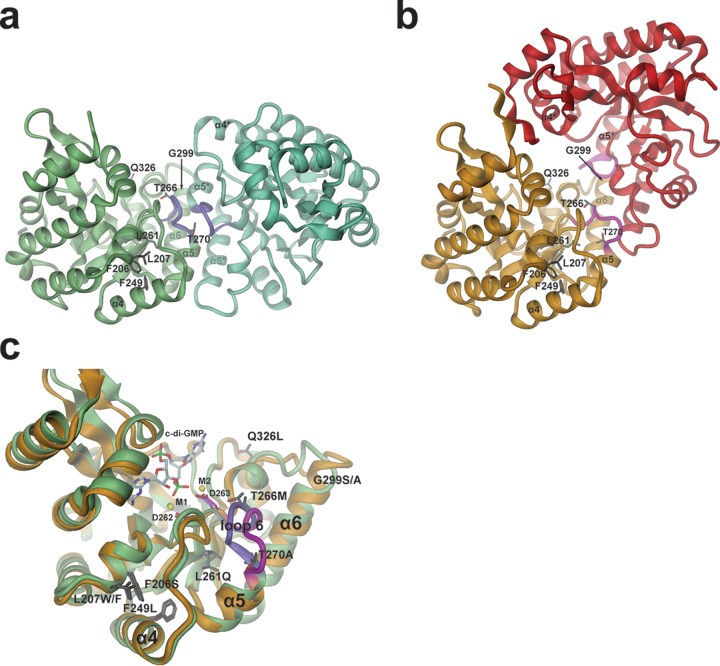
FIG 7.
Model for PdeL phosphodiesterase activation by suppressor mutations. (a and b) Two distinct EAL dimer structures are shown as obtained recently by X-ray crystallography (18). The sites of suppressor mutations are shown in full. The relative orientations of the dimers have been adjusted such that in each panel the left monomer is seen in the same orientation and position. In each case, the two monomers are distinguished by different colors. Purple and magenta coloring highlights the loop 6 region. (a) Structure of the canonical “open” PdeL EAL dimer as determined in the presence of magnesium (PDB code 4LYK). (b) Structure of the “closed” PdeL EAL dimer as determined in the presence of c-di-GMP/Ca2+ (PDB code 4LJ3). (c) Comparison of the PdeL EAL monomer structures of the “open” and “closed” dimers. Colors are the same as those shown in panels a and b (green with loop 6 in purple, “open” dimer; gold with magenta loop, “closed” dimer). In addition to the mutation sites (dark gray residues), the substrate c-di-GMP, the calcium ions M1 and M2, and metal-coordinating aspartates 262 and 263 at the end of β-strand 5 are highlighted. The latter precedes loop 6 and has been implicated in catalysis regulation (18).