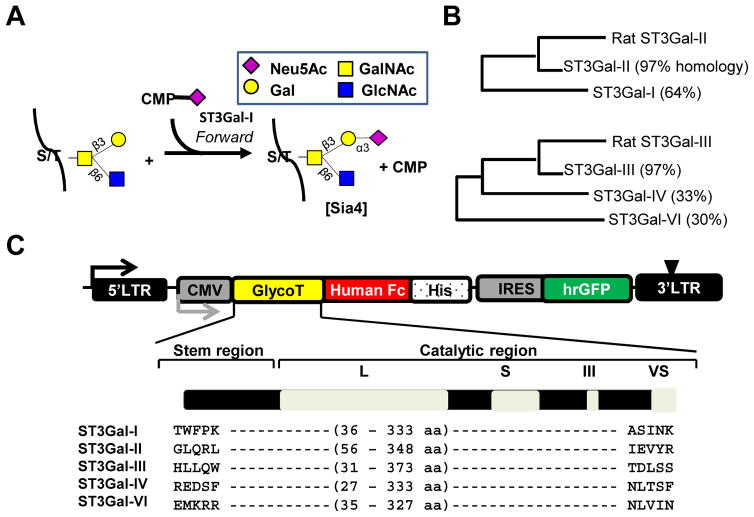
Figure 1. Sialyltransferase expression and activity.
A. Sialylation mediated by human ST3Gal-I results in the transfer of sialic acid from the donor, CMP-Neu5Ac, to the glycoprotein acceptor. Glycans are represented using the Consortium of Functional Glycomics nomenclature (http://www.functionalglycomics.org/static/consortium/Nomenclature.shtml). B. Similarity in the sequence of the catalytic domains of rat and human ST3Gals analyzed using ClustalW2 (http://www.ebi.ac.uk/Tools/msa/clustalw2/). C. pCSCG vector expresses human α2,3 sialyltransferases, ST3Gal-I, -II, -III, -IV and -VI, as fusion protein with C-terminal Fc and his-tag. Amino acids expressed are annotated. Expression of IRES-GFP reporter protein confirms stable enzyme synthesis in mammalian cells.