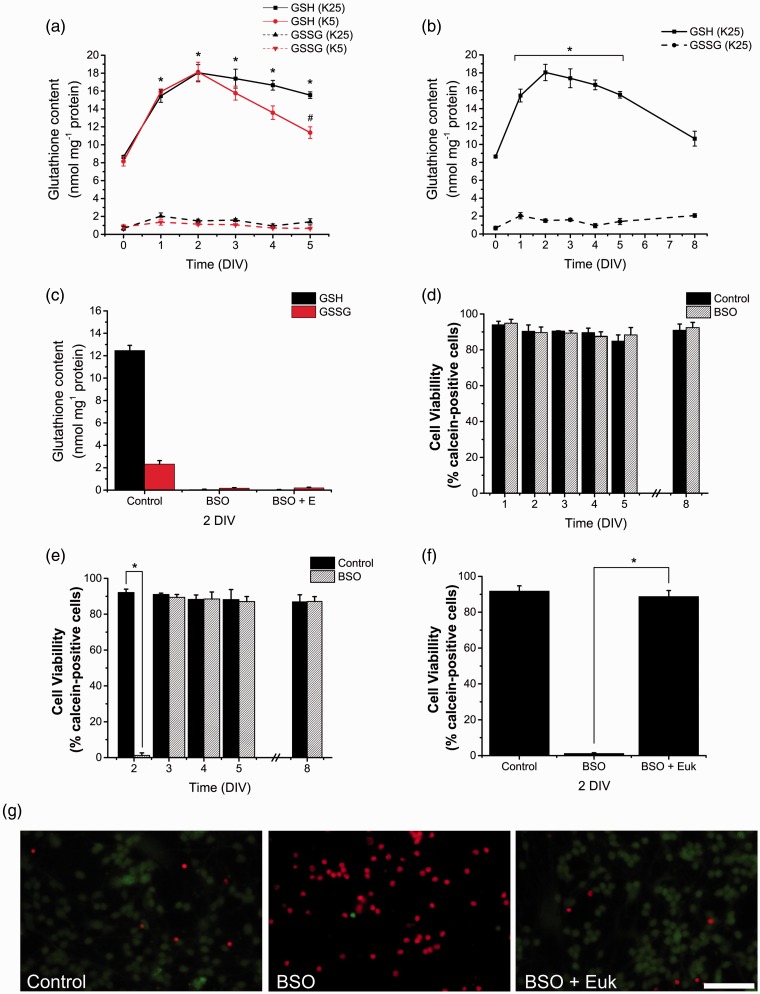
Figure 2.
Glutathione is differentially produced and is necessary for CGN survival. (a, b) Reduced glutathione (GSH) and oxidized glutathione (GSSG) content were determined in CGN grown in K25 and K5 from 0 to 8 DIV by a modification of the Tietze recycling assay as detailed in Methods. (a) * is significantly different from K25 at 0 DIV (p < .001, ANOVA, n = 4). # is significantly different from K25 at 5 DIV (p < .001, ANOVA, n = 4). Data are mean ± SEM. (b) * is significantly different from K25 at 0 DIV (p < .001, ANOVA, n = 4). Data are mean ± SEM. (c) GSH and GSSG were determined in CGN grown in K25 at 2 DIV and treated with BSO (100 µM) for 48 hr and Euk-134 (10 µM) for 24 hr. BSO treatments reduced the levels of GSH and GSSG (p < .05, ANOVA nonparametric test, n = 5). Data are mean ± SEM. (d to f) Cell viability was determined by calcein and propidium iodide. Data are expressed as the percentage of calcein-positive cells from the total number of cells, which was estimated as the sum of calcein-positive cells plus propidium iodide-positive cells. (d) Cell viability was determined in CGN grown in K25 and treated with BSO (100 µM) for 24 hr at 1, 2, 3, 4, 5, and 8 DIV (no statistical differences were found, ANOVA, n = 4). (e) Cell viability was determined in CGN grown in K25 and treated with BSO (100 µM) for 48 hr at 2, 3, 4, 5, and 8 DIV. * is significantly different from Control at 2 DIV (*p < .001, ANOVA, n = 4). (f) Cell viability was determined in CGN grown in K25 at 2 DIV and treated with BSO (100 µM) for 48 hr and Euk-134 (10 µM) for 24 hr. * is significantly different from BSO at 2 DIV (p < .001, ANOVA, n = 4). Data are mean ± SEM. (g) Representative micrographs of CGN grown in K25 and treated with BSO (100 µM) for 48 hr and Euk-134 (10 µM) for 24 hr. Calcein-positive cells are marked in green and propidium iodide-positive cells are marked in red (scale bar, 100 µm). ANOVA = analysis of variance; BSO = buthionine sulphoximine; CGN = cerebellar granule neurons; DIV = days in vitro.