Abstract
Background
Gallium-67 scintigraphy has been suggested as a noninvasive method to diagnose acute interstitial nephritis (AIN). However, its diagnostic performance and usefulness remain controversial.
Methods
We retrospectively reviewed the charts of 76 patients who underwent gallium-67 scintigraphy for a suspicion of AIN. Patients were classified based on kidney biopsy and/or clinical probability of AIN. Gallium-67 scintigraphy results were reinterpreted blindly using both posterior planar and single photon emission computed tomography (SPECT) imaging. Intensity of radioisotope uptake in the kidney was graded from 0 to 5.
Results
The diagnosis of AIN was confirmed in 23 patients and excluded in 44. Nine patients with an uncertain diagnosis were excluded from subsequent analysis. A gallium-67 kidney uptake cutoff of 1 gave a negative predictive value of 100%, whereas a cutoff of 5 had an excellent specificity and positive predictive value for the diagnosis of AIN. When using a cutoff of 3, which had previously been used in the literature, we obtained a sensitivity of 61% and a specificity of 75% with posterior planar imaging. The results of both SPECT and posterior planar imaging modalities were comparable.
Conclusions
Gallium-67 scintigraphy may be of interest in patients with a clinical suspicion of AIN, especially in those who are unable to undergo kidney biopsy. However, results need to be interpreted with caution and depend on the intensity of gallium-67 kidney uptake.
Keywords: gallium scintigraphy, interstitial nephritis
Introduction
Acute interstitial nephritis (AIN) accounts for up to 15% of kidney biopsies performed to investigate unexplained acute kidney injury (AKI) [1, 2]. Immunoallergic drug reaction is by far the most common cause of AIN, accounting for 75–90% of cases [3]. Recognition of AIN is important, since early withdrawal of the causative drug and possibly corticosteroids can lead to recovery of kidney function [1, 4, 5]. Extra-renal manifestations of disease such as fever, rash or eosinophilia are present in only 25–35% of patients with drug-induced AIN, making the clinical diagnosis often difficult [1, 5]. Limited reliability of laboratory tests renders the diagnosis difficult, and in most cases, kidney biopsy is required for a definitive diagnosis. However, kidney biopsy incurs risks of bleeding and may be contraindicated or difficult in some instances [6].
Thus, noninvasive diagnostic tests for AIN would be helpful for patients who are not candidates for kidney biopsy. Gallium-67 scintigraphy has been suggested as a safe and noninvasive technique to aid in the diagnosis of AIN [7–10]. Gallium-67 is a radiotracer that binds to inflammatory proteins such as lactoferrin [10] and thus pools up at the sites of inflammation. Gallium-67 scintigraphy, following intravenous injection of gallium-67, is commonly used for detection of sites with inflammation or infection.
Wood et al. [7] reported more than three decades ago kidney uptake of gallium-67 in three patients with AIN. These results were corroborated by a large series showing a 100% sensitivity of gallium-67 scintigraphy in patients with biopsy-proven AIN [8]. More recently, gallium-67 scintigraphy was shown to be very effective in differentiating acute tubular necrosis from AIN in rats [10]. Nevertheless, other studies challenged the sensitivity [11, 13, 14] and the specificity [8, 12] of gallium-67 scintigraphy.
In order to better delineate the efficacy of gallium-67 scintigraphy in diagnosing AIN, we conducted a retrospective study on 76 patients who underwent a gallium-67 scintigraphy for a suspicion of interstitial nephritis.
Materials and methods
Study design and data collection
We retrospectively reviewed the charts of 76 patients who underwent a gallium-67 scintigraphy for a suspicion of AIN between 29 January 2010 and 21 March 2014. The patients were enrolled in three different hospital centers (Notre-Dame, St-Luc and Hôtel-Dieu) that are part of the University of Montreal Health Care Center (CHUM). Patients were identified through the database of the Nuclear Medicine Department. Full medical charts were thoroughly reviewed by F.G. to collect clinical data. The study was approved by the ethics committee of our institution.
Outcome
We divided patients into three categories based on the probability of AIN: very probable or confirmed (Group 1), highly unlikely or excluded (Group 2) and uncertain (Group 3). Then, G.B. reviewed clinical data collected by F.G. in a blinded manner so as to not know the gallium-67 scintigraphy results when proceeding to classification of patients.
Group 1 contained patients with biopsy-proven interstitial nephritis and/or AKI associated with signs of immunoallergy that began after introduction of medication and resolved after cessation of medication, and/or a chronology of AKI associated with the introduction of a new medication and resolving after the cessation of the medication introduced highly in favor of a diagnosis of AIN. Signs of immunoallergy included eosinophilia (>0.9 × 109/L), hepatitis (AST and ALT >1.5 times the normal values), urticarial rash or arthritis, without alternative explanation. Patients with clinical features or evidence suggesting any other cause of kidney injury, such as factors associated with prerenal AKI (hypotension, dehydration), proteinuria >1.5 g/day or nephrotoxic medication, were excluded from Group 1. Group 2 contained patients with a kidney biopsy excluding interstitial nephritis and/or clinical features making the diagnosis of AIN highly unlikely. Patients without kidney biopsy were included in this group only if they had no signs of immunoallergy (as described above) and if kidney dysfunction was likely explained by factors other than immunoallergy or any other cause of AIN. Group 3 were patients who did not have criteria to fit in either Group 1 or 2 and where AIN could neither be confirmed nor excluded beyond reasonable doubt.
Gallium-67 scintigraphy
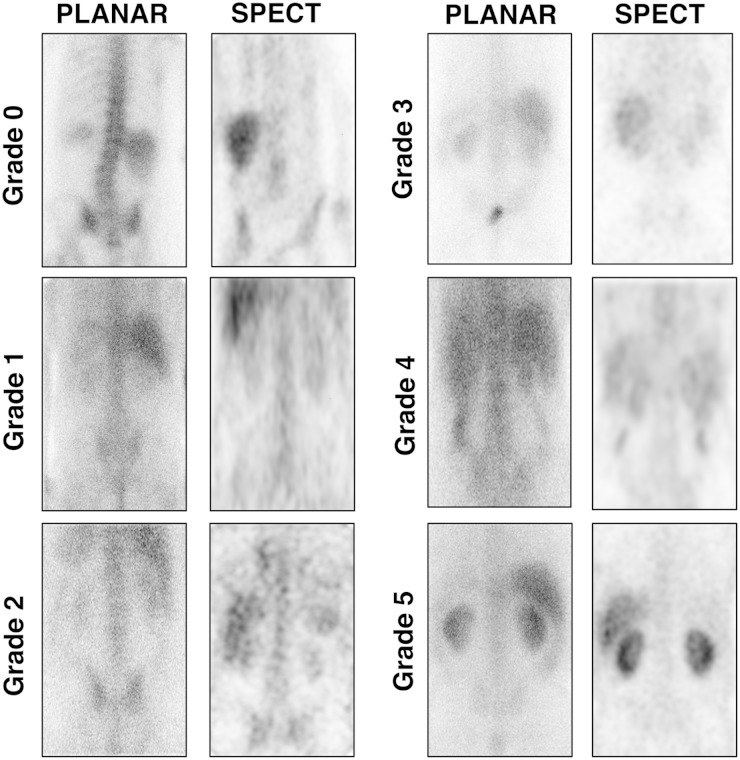
Gallium-67 citrate is an isotope with a half-life of 78 h [7]. Gallium-67 citrate was injected in patients on Day 1 with images taken by gamma camera at 48, 72 or 96 h after injection. The dose of gallium-67 citrate administered to each patient varied between 5 and 10 mCi (185–370 MBq). Single photon emission computed tomography (SPECT) and posterior planar imaging were used to analyze data. The intensity of radioisotope uptake in the kidneys was graded on a scale of 0 to 5 and compared with the intensity of the liver and spine—Grade 0: no kidney uptake; 1: kidney uptake lower than spine; 2: kidney uptake equal to spine; 3: kidney uptake higher than spine but lower than liver; 4: kidney uptake equal to liver; and 5: kidney uptake higher than liver. All gallium-67 scintigraphies were reinterpreted using both SPECT and planar imaging by a specialist in nuclear medicine (M.L.) and blinded to patients' clinical data.
Statistics
Quantitative data are expressed as median and interquartile range (25th and 75th percentiles), and qualitative data as number and percentages. Groups were compared using the χ2 test or Fisher's exact test when appropriate for categorical variables, and using the Mann–Whitney U-test for continuous variables. Sensitivity, specificity, positive predictive values and negative predictive values for gallium-67 scintigraphy using both SPECT and planar analysis were calculated using different cutoff values. Confidence intervals were calculated using Wilson score intervals. Receiver operating characteristic (ROC) curves for both SPECT and planar methods were produced using sensitivity and 1-specificity. Statistical analyses were performed with SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Patient characteristics
The population consisted of 76 patients with kidney dysfunction for whom a gallium-67 scintigraphy had been ordered to investigate the possibility of interstitial nephritis. As described in the Materials and methods, patients were classified into three categories based on the probability of interstitial nephritis: very probable or confirmed (Group 1), highly unlikely or excluded (Group 2) and uncertain (Group 3). Twenty-three patients comprised Group 1, 44 patients Group 2 and 9 patients Group 3. Patients with an uncertain diagnosis (Group 3) were excluded from the subsequent analysis.
In Group 1 patients, AIN was diagnosed based on kidney biopsy in 8 out of 23 (34.7%) patients, and on clinical features and laboratory findings in 15 out of 23 (65.2%) patients. AIN could be attributed to drug allergy (n = 19, 82.6%), natural products (n = 1, 4.3%), Sjögren (n = 1, 4.3%) and remained of uncertain etiology in two patients (8.7%), with several drugs suspected in one of them (Table 1). One patient had lupus, although nephritis was likely induced by pantoprazole.
Table 1.
Final diagnosis in study patients
| Group 1: AIN | Group 2: other diagnosis | |
|---|---|---|
| Number of patients | 23 | 44 |
| Patients with kidney biopsy (%) | 9 (39.1) | 12 (27.3) |
| Final diagnosis (%) | ||
| AIN | 23 (100) | |
| Drug induced | 19 (82.6) | |
| Natural products | 1 (4.3) | |
| Sjögren | 1 (4.3) | |
| Uncertain | 2 (8.7) | |
| Other diagnosis—no AIN | ||
| Histologic diagnosis | ||
| Glomerulosclerosis or chronic TI changes | 5 (11.4) | |
| Glomerulonephritis | 5 (11.4) | |
| Hypertensive nephropathy | 1 (2.3) | |
| Normal kidney | 1 (2.3) | |
| Clinical diagnosis | ||
| Stable CKD | 4 (9.1) | |
| Transient and mild increase of SCr | 3 (6.8) | |
| Prerenal AKI | 7 (15.9) | |
| Contrast nephropathy | 3 (6.8) | |
| Acute tubular necrosis | 5 (11.4) | |
| Cardiorenal syndrome | 3 (6.8) | |
| Multifactorial | 4 (9.1) | |
| Postrenal AKI | 1 (2.3) | |
| Uveitis with normal kidney function | 1 (2.3) | |
| Isolated proteinuria | 1 (2.3) | |
Group 1: patients with confirmed or very probable AIN; Group 2: AIN excluded or highly unlikely. Quantitative data are expressed as median (25th–75th percentiles) and qualitative data as numbers (%).
SCr, serum creatinine level; TI, tubulointerstitial.
In 12 out of 44 (27%) patients in Group 2, AIN was excluded by kidney biopsy, which demonstrated other diagnoses (Table 1). In the subgroup of patients in Group 2 who did not have a biopsy (n = 32, 73%), AIN could be excluded beyond reasonable doubt, since the clinical presentation and the evolution after gallium-67 scintigraphy were not suggestive of AIN, and kidney dysfunction could be explained by one or several factors (Table 1). Two patients, one with uveitis and one patient with isolated mild proteinuria, had normal kidney function, making AIN very unlikely. In four (9%) cases, there was in fact no acute increase in serum creatinine level and these patients had stable chronic kidney disease (CKD). In three (6.8%) patients, serum creatinine increased only mildly and spontaneously returned to the baseline level right after the gallium-67 scintigraphy, which was not compatible with a diagnosis of AIN.
Clinical features of Group 1 and 2 patients are summarized in Table 2. Extra-renal disease potentially associated with interstitial nephritis (Sjögren, sarcoidosis or lupus) was present in two (8.7%) patients in Group 1 and in six (13.63%) patients in Group 2. One (4.3%) patient in Group 1 and four (9.1%) in Group 2 were kidney transplant recipients (P = 0.71). We analyzed the frequency of manifestations associated with allergy, especially eosinophilia, rash, hepatitis and arthritis (in the absence of other etiology). Seven out of 23 (30.4%) patients in Group 1 had eosinophilia compared with 2 out of 44 (4.5%) in Group 2 (P < 0.01). Skin rash was present in five patients (21.7%) in Group 1 and in three patients (6.8%) in Group 2 (P = 0.12). Hepatitis was present in five (21.7%) patients in Group 1 and in none of the patients in Group 2 (P < 0.01).
Table 2.
Clinical characteristics of study subjects at the time of gallium-67 scintigraphy
| Group 1: AIN | Group 2: other diagnosis | P-value | |
|---|---|---|---|
| Number of patients | 23 | 44 | |
| Male (%) | 9 (39) | 20 (45) | 0.79 |
| Age (years) | 65 (51.5–72) | 65 (51–72) | 0.61 |
| Baseline serum creatinine (µmol/L) | 72 (62–96) | 114 (84–133) | 0.29 |
| Serum creatinine at gallium-67 scintigraphy (µmol/L) | 189 (124–370) | 158 (132–216) | 0.2 |
| Kidney transplant recipient | 1 (4.3) | 5 (11.3) | 0.66 |
| Extra-renal disease (%) | 1 (4.3) | 4 (9.1) | 0.71 |
| Sjögren | 1 | 3 | 1 |
| Sarcoidosis | 0 | 2 | 0.54 |
| Lupus | 1 | 1 | 1 |
| Proteinuria (g/mmol) | 0.06 (0.03–0.18) | 0.15 (0.07–0.23) | 0.1 |
| Leucocyturia (%) | 10 (39.1) | 14 (31.8) | 0.42 |
| Microhematuria (%) | 8 (34.8) | 17 (38.6) | 0.8 |
| Granular casts (%) | 1/5 (20) | 0 /10 (0) | 0.2 |
| Urinary eosinophils (%) | 2/13 (15) | 1/23 (4.3) | 0.54 |
| Extrarenal signs of allergy (%) | |||
| Rash | 5 (21.7) | 3 (6.8) | 0.12 |
| Eosinophilia | 7 (30.4) | 2 (4.5) | 0.006 |
| Hepatitis | 5 (21.7) | 0 | 0.0064 |
| Arthritis | 1 (4.3) | 1 (2.3) | 1 |
Quantitative data are expressed as median (25th–75th percentiles) and qualitative data as numbers (%).
Performance characteristics of gallium-67 scintigraphy
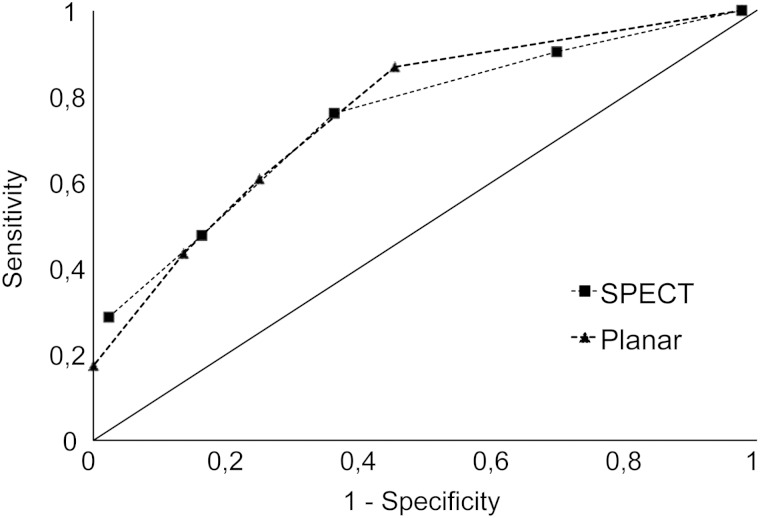
In order to evaluate the diagnostic value of gallium-67 scintigraphy for AIN, the intensity of radioisotope uptake in the kidneys was compared between Group 1 and Group 2 patients. Gallium-67 scintigraphy images were reinterpreted using both SPECT and planar imaging and graded from 0 to 5 (Figure 1). Out of the 67 patients included for analysis, three patients had planar imaging alone, whereas the others (n = 64) had both SPECT and planar imaging performed. Gallium-67 scintigraphy is usually considered positive if posterior planar imaging demonstrates a kidney uptake equal to or higher than that of the liver [11] or the spine [8]. We investigated whether using different cutoffs could improve diagnostic performance. Results varied significantly depending on the analysis method chosen and the grade taken as cutoff value (Table 3). All except one patient had Grade 1 or higher uptake of gallium-67. Using Grade ≥2 as cutoff, sensitivity was 0.87 and negative predictive value (NPV) 0.89, but specificity and positive predictive value (PPV) were only 0.55 and 0.5, respectively. Grade 5 was associated with specificity and PPV of 100%, but also with lower sensitivity (0.17) and NPV (0.7). Results using SPECT and posterior planar were comparable, as illustrated by ROC curves (Figure 2). The area under the ROC curve was 0.7492 for planar scintigraphy and 0.7514 for SPECT.
Fig. 1.
Grading of gallium-67 uptake in the kidneys by SPECT (coronal view) and planar scintigraphy. Images were obtained at 72 h (Grades 1, 3, 4 and 5) and 92 h (Grades 0 and 2).
Table 3.
Evaluation of diagnostic performance of gallium-67 scintigraphy using SPECT or posterior planar imaging, for the assessment of AIN
| Diagnostic criterion | Group 1: AIN | Group 2: other diagnosis | SEN (95% CI) | SPEC (95% CI) | PPV (95% CI) | NPV (95% CI) |
|---|---|---|---|---|---|---|
| Posterior planar | n = 23 | n = 44 | ||||
| Grade ≥1 | 23 | 43 | 1 (0.86–1) | 0.02 (0–0.12) | 0.35 (0.25–0.47) | 1 (0.21–1) |
| Grade ≥2 | 20 | 20 | 0.87 (0.68–0.95) | 0.55 (0.40–0.68) | 0.5 (0.35–0.65) | 0.89 (0.72–0.96) |
| Grade ≥3 | 14 | 11 | 0.61 (0.41–0.78) | 0.75 (0.61–0.85) | 0.56 (0.39–0.75) | 0.79 (0.65–0.88) |
| Grade ≥4 | 10 | 6 | 0.43 (0.26–0.63) | 0.86 (0.73–0.94) | 0.62 (0.37–0.81) | 0.75 (0.61–0.84) |
| Grade ≥5 | 4 | 0 | 0.17 (0.07–0.37) | 1 (0.92–1) | 1 (0.51–1) | 0.70 (0.58–0.80) |
| SPECT | n = 21 | n = 43 | ||||
| Grade ≥1 | 21 | 42 | 1 (0.84–1) | 0.02 (0–0.12) | 0.33 (0.23–0.46) | 1 (0.21–1) |
| Grade ≥2 | 19 | 30 | 0.90 (0.71–0.97) | 0.30 (0.19–0.45) | 0.39 (0.26–0.53) | 0.87 (0.62–0.96) |
| Grade ≥3 | 16 | 16 | 0.76 (0.55–0.89) | 0.64 (0.49–0.76) | 0.5 (0.34–0.66) | 0.85 (0.69–0.93) |
| Grade ≥4 | 10 | 7 | 0.48 (0.28–0.68) | 0.84 (0.70–0.92) | 0.59 (0.36–0.78) | 0.77 (0.63–0.86) |
| Grade ≥5 | 6 | 1 | 0.29 (0.14–0.5) | 0.98 (0.88–1) | 0.86 (0.49–0.97) | 0.74 (0.61–0.83) |
SEN, sensitivity; SPEC, specificity; CI, confidence interval.
Fig. 2.
ROC illustrating the diagnostic performance of posterior planar and SPECT imaging for the assessment of AIN.
When gallium-67 scintigraphy was performed, exposure to the causal agent was still ongoing in 11 out of 20 (55%) patients with AIN induced by drug or natural products. In other patients, the median delay between the interruption of exposure and gallium-67 scintigraphy was 1 day [interquartile range (IQR): 1–5 days]. Posterior planar grade was similar in eight patients who stopped the causative drug >48 h before gallium-67 scintigraphy (median Grade 3, IQR: 2–4) and in 12 patients who were still receiving the causative drug in the 48 h preceding gallium-67 scintigraphy (median Grade 2, IQR: 2–4) (P = 0.75).
Results of gallium-67 scintigraphy in the subgroup of patients with kidney biopsy
Twenty patients of our study underwent a kidney biopsy based on clinical suspicion of interstitial nephritis (Table 4). The results of posterior planar and SPECT imaging were similar in these patients to those observed in the whole cohort. Among patients with biopsy-proven AIN (n = 8, Patients 1–8 in Table 4), gallium-67 scintigraphy showed Grade 4 or 5 positivity in four of them (44.4%) using planar or SPECT imaging.
Table 4.
Results of gallium-67 scintigraphy in the subgroup of patients with kidney biopsy
| Patient | Posterior planar grade | SPECT grade | Comment | Time intervala (days) |
|---|---|---|---|---|
| AIN (Group 1) | ||||
| 1 | 1 | 1 | Drug-induced AIN (cloxacilline) | 11 |
| 2 | 2 | 3 | Drug-induced AIN (ranitidine) | 67 |
| 3 | 3 | 3 | Sjögren syndrome | 159 |
| 4 | 4 | 4 | Drug induced AIN (pantoprazole) | 19 |
| 5b | 4 | 5 | AIN of uncertain etiology | 65 |
| 6 | 4 | 4 | Drug-induced AIN | 4 |
| 7 | 5 | 5 | AIN induced by natural products | 4 |
| 8 | 5 | 5 | Drug-induced AIN (ciprofloxacin) | 26 |
| Other diagnosis (Group 2) | ||||
| 9 | 1 | 1 | Mild chronic TI changes | 54 |
| 10b | 1 | 1 | Glomerulosclerosis, chronic TI changes | 40 |
| 11 | 1 | N/A | Pauci-immune crescentic GN | 7 |
| 12 | 1 | 1 | Pauci-immune crescentic GN, interstitial infiltrates | 23 |
| 13 | 1 | 1 | Glomerulosclerosis, chronic TI changes | 10 |
| 14 | 1 | 2 | Hypertensive nephropathy | 24 |
| 15 | 2 | 2 | Membranoproliferative GN | 23 |
| 16 | 2 | 2 | No significant histologic changes | 28 |
| 17b | 3 | 3 | IgA nephropathy | 37 |
| 18 | 4 | 4 | Pauci-immune crescentic GN | 8 |
| 19 | 4 | 4 | Glomerulosclerosis | 105 |
| 20 | 4 | 4 | Mild tubular atrophy | 48 |
TI, tubulointerstitial; GN, glomerulonephritis; N/A, Not available.
aTime interval between gallium scintigraphy and kidney biopsy.
bKidney transplant recipient.
In 12 cases (Patients 9–20 in Table 4), interstitial nephritis was excluded by the kidney biopsy. In Group 2 patients, the course of kidney dysfunction was often subacute or chronic and there was some delay between gallium-67 scintigraphy and kidney biopsy. However, the clinical presentation and evolution of kidney function did not suggest, in any of patients, that interstitial nephritis could have been present earlier and resolved before the biopsy. In one patient (11.4%), the interstitium showed inflammatory infiltrates, although the predominant pattern was crescentic glomerulonephritis (Patient 13). In three patients (25%), gallium-67 scintigraphy showed Grade 4 positivity, but Grade 5 positivity was not observed in patients without interstitial nephritis. Eight patients (75%) showed only Grade 1 or Grade 2 uptake.
Discussion
Although the use of gallium-67 scintigraphy for diagnosis of AIN is not a universal practice and remains controversial, this technique has often been used at our institution, as part of the workup for assessment of the etiology of kidney dysfunction. To further define the role of kidney scintigraphy, we retrospectively studied the results of gallium-67 scans performed in patients with AIN or an alternative diagnosis. All gallium scintigraphy results were reinterpreted blindly. Studies in the literature are discordant when it comes to grading the intensity of gallium uptake by the kidney and to defining a positive or negative result [8, 11]. Our study is novel in that we further evaluated the role of grading kidney uptake by distinguishing five classes of uptake and by comparing SPECT and planar imaging. Our results indicate that the performance of planar and SPECT imaging were similar, as there was a concordance of grading in the majority of cases. We found an overlap between results of patients with AIN and those with other diagnoses. Thus, using one unique cutoff to define a positive or negative exam may not be appropriate. We tried to determine a kidney uptake threshold under which the diagnosis would be very improbable and a cutoff over which the diagnosis would be very probable. Grades 0 and 1 had a good NPV, and allowed exclusion of AIN with a strong probability. Grade 5 had an excellent PPV, but low sensitivity. Grade 4 had a good specificity (85%) with a slightly lower PPV than Grade 5. In between both values lay a gray zone, with a PPV and NPV that may be inadequate for an important diagnosis such as AIN.
Our results show the interest of grading results and confronting the grade to clinical probability.
A few studies have previously evaluated the use of gallium scintigraphy in the diagnosis of AIN although most of them were performed two to three decades ago [7–9, 11, 13, 16]. The largest study to date was performed by Linton et al. [8], and involved a cohort of 44 patients with biopsy-proven kidney disease. The sensitivity of gallium-67 scintigraphy was 100% in 11 patients with AIN confirmed by biopsy. In two smaller studies, the reported sensitivity was only 58% [11] and 69% [13]. Furthermore, gallium-67 scintigraphy was shown to have a specificity of 50–84% with positive results in patients with various inflammatory conditions [8, 12, 15]. The interpretation of these studies was limited as they lacked power and the definition of a positive gallium was not always standardized and often not well documented. These studies did not explore the interest of grading different cutoffs to improve diagnostic performances; all previous studies were solely performed using planar imaging.
Our study also has limitations inherent to retrospective studies. Not all patients underwent the gold standard of kidney biopsy and when performed, biopsy was often delayed after gallium-67 scintigraphy. Kidney biopsy was not performed or was delayed for multiple reasons, including frailty and bleeding disorders; in many cases, the clinician was able to confidently emit an alternative diagnosis without needing a biopsy in light of the evolution of the patient's kidney function (prerenal cause, acute tubular necrosis, cardiorenal syndrome, etc.). In some patients with AIN, biopsy was not performed at first, but only later due to the lack of recovery of kidney function. For the purpose of our study, patients were classified in a blinded manner without knowing the results of the gallium scintigraphy based on a detailed clinical revision of charts, which limited classification bias. Furthermore, the results of gallium scintigraphies in the subgroup of patients with kidney biopsies were concordant with results observed in the whole cohort, which is reassuring on the classification of patients with a clinical diagnosis.
Our data did not allow us to evaluate the relationship between the intensity of gallium-67 uptake and the degree of interstitial infiltration on kidney biopsy. It remains unknown why some patients with interstitial nephritis showed only low or intermediate kidney uptake. This was unlikely to be explained by resolution of interstitial inflammation when gallium-67 scintigraphy was performed, since kidney dysfunction was persisting at the time of gallium-67 scintigraphy and we found no difference in gallium-67 uptake between patients who stopped the causative drug >48 h before gallium-67 scintigraphy and those who did not. It is also unclear why gallium uptake appeared relatively high (Grade 4) in a few patients with diagnosis other than interstitial nephritis. This may be related to interstitial infiltrates that can be seen in numerous cases of acute or chronic disease other than interstitial nephritis (e.g. glomerulonephritis and renal fibrosis).
Despite these limitations, our study suggests that gallium scintigraphy may be useful in patients who are not candidates for a kidney biopsy, although it does not have the specificity and sensitivity to replace kidney biopsy for the diagnosis of AIN. Larger studies including more biopsy-proven AIN versus biopsy-ruled out controls are needed to further evaluate the role of gallium scintigraphy in the diagnosis of AIN.
Authors’ contributions
All authors contributed to the analysis and interpretation of data, writing and revision of manuscript, and final approval of manuscript.
Conflict of interest statement
All authors state that they have no conflicts of interest to declare. The results presented in this paper have not been published previously in whole or part.
References
- 1.Clarkson MR, Giblin L, O'Connell FP, et al. Acute interstitial nephritis: clinical features and response to corticosteroid therapy. Nephrol Dial Transplant 2004; 19: 2778–2783 [DOI] [PubMed] [Google Scholar]
- 2.Neilson EG. Pathogenesis and therapy of interstitial nephritis. Kidney Int 1989; 35: 1257–1270 [DOI] [PubMed] [Google Scholar]
- 3.Praga M, Gonzalez E. Acute interstitial nephritis. Kidney Int 2010; 77: 956–961 [DOI] [PubMed] [Google Scholar]
- 4.Praga M, Sevillano A, Aunon P, et al. Changes in the aetiology, clinical presentation and management of acute interstitial nephritis, an increasingly common cause of acute kidney injury. Nephrol Dial Transplant 2015; 30: 1472–1479 [DOI] [PubMed] [Google Scholar]
- 5.Gonzalez E, Gutierrez E, Galeano C, et al. Early steroid treatment improves the recovery of renal function in patients with drug-induced acute interstitial nephritis. Kidney Int 2008; 73: 940–946 [DOI] [PubMed] [Google Scholar]
- 6.Brachemi S, Bollee G. Renal biopsy practice: what is the gold standard? World J Nephrol 2014; 3: 287–294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wood BC, Sharma JN, Germann DR, et al. Gallium citrate Ga 67 imaging in noninfectious interstitial nephritis. Arch Intern Med 1978; 138: 1665–1666 [PubMed] [Google Scholar]
- 8.Linton AL, Richmond JM, Clark WF, et al. Gallium67 scintigraphy in the diagnosis of acute renal disease. Clin Nephrol 1985; 24: 84–87 [PubMed] [Google Scholar]
- 9.Shibasaki T, Ishimoto F, Sakai O, et al. Clinical characterization of drug-induced allergic nephritis. Am J Nephrol 1991; 11: 174–180 [DOI] [PubMed] [Google Scholar]
- 10.Joaquim AI, Mendes GE, Ribeiro PF, et al. Ga-67 scintigraphy in the differential diagnosis between acute interstitial nephritis and acute tubular necrosis: an experimental study. Nephrol Dial Transplant 2010; 25: 3277–3282 [DOI] [PubMed] [Google Scholar]
- 11.Graham GD, Lundy MM, Moreno AJ. Failure of gallium-67 scintigraphy to identify reliably noninfectious interstitial nephritis: concise communication. J Nucl Med 1983; 24: 568–570 [PubMed] [Google Scholar]
- 12.Rossert J. Drug-induced acute interstitial nephritis. Kidney Int 2001; 60: 804–817 [DOI] [PubMed] [Google Scholar]
- 13.Koselj M, Kveder R, Bren AF, et al. Acute renal failure in patients with drug-induced acute interstitial nephritis. Ren Fail 1993; 15: 69–72 [DOI] [PubMed] [Google Scholar]
- 14.Handa SP. Drug-induced acute interstitial nephritis: report of 10 cases. CMAJ 1986; 135: 1278–1281 [PMC free article] [PubMed] [Google Scholar]
- 15.Lavender JP, Lowe J, Barker JR, et al. Gallium 67 citrate scanning in neoplastic and inflammatory lesions. Br J Radiol 1971; 44: 361–366 [DOI] [PubMed] [Google Scholar]
- 16.Pagniez D, MacNamara E, Beuscart R, et al. Renal gallium scintigraphy and acute drug-induced interstitial nephritis. Nephrologie 1987; 8: 70. [PubMed] [Google Scholar]