Abstract
Background
Arteriovenous fistula (AVF) failure to mature (FTM) rates contribute to excessive dependence on central venous catheters for haemodialysis. Choosing the most appropriate vascular access site for an individual patient is guided largely by their age, co-morbidities and clinical examination. We investigated the clinical predictors of AVF FTM in a European cohort of patients and applied an existing clinical risk prediction model for AVF FTM to this population.
Methods
A prospective cohort study was designed that included all patients undergoing AVF creation between January 2009 and December 2014 in a single centre (Belfast City Hospital) who had a functional AVF outcome observed by March 2015.
Results
A total of 525 patients had a functional AVF outcome recorded and were included in the FTM analysis. In this cohort, 309 (59%) patients achieved functional AVF patency and 216 (41%) patients had FTM. Female gender [P < 0.001, odds ratio (OR) 2.03 (CI 1.37–3.02)] and lower-arm AVF [P < 0.001, OR 4.07 (CI 2.77–5.92)] were associated with AVF FTM. The Lok model did not predict FTM outcomes based on the associated risk stratification in our population.
Conclusions
In this European study, female gender was associated with twice the risk of AVF FTM and a lower-arm AVF with four times the risk of FTM. The FTM risk prediction model was not found to be discriminative in this population. Clinical risk factors for AVF FTM vary between populations; we would recommend that units investigate their own clinical predictors of FTM to maximize AVF functional patency and ultimately survival in dialysis patients. Clinical predictors of AVF FTM may not be sufficient on their own to improve vascular access functional patency rates.
Keywords: arteriovenous fistula, dialysis, failure to mature, vascular access
Introduction
United Kingdom renal registry data report that the relative risk of death in individuals on renal replacement therapy ages 30–34 years is 18 times that of the general population, and in patients >85 years it is 2.5 times that of the general population [1]. The use of arteriovenous fistulas (AVFs) compared with central venous catheters (CVCs) for vascular access has been associated with increased haemodialysis patient survival [2–4]. Initial use of AVFs for haemodialysis varies widely, with an incident AVF use rate of 14% in the USA compared with 32% in Europe [5, 6]. It is well recognized that high rates of failure to mature (FTM) limit widespread adoption of AVFs for vascular access [7].
Although improved AVF outcomes have been associated with strategies such as routine preoperative ultrasound mapping [8], which identifies optimal blood vessels prior to AVF creation, these services are not always readily available [9] or indeed may not influence surgical decision-making [10]. Thus, in the ‘real-world’ setting, the renal team often relies on clinical characteristics, such as the patient's age and burden of co-morbidities, which may influence vascular anatomy and survival, when deciding on the best vascular access strategy for an individual patient.
A survey of Canadian and American nephrologists found that patients were more likely to be referred for AVF creation if they were <65 years old and had minimal co-morbidities or no history of failed vascular access attempts [11]. These findings were echoed in an international survey of 134 surgeons examining preference and practice in haemodialysis vascular access creation; increased co-morbidities and previous failed vascular access procedures were seen as potential deterrents to AVF creation [10]. Of interest, 42% of surgeons stated that they had no absolute contraindications to AVF creation.
Several studies in differing populations have identified a variety of clinical factors associated with AVF FTM, such as age ≥65 years [12], female gender [13], uraemia [14], diabetes, peripheral vascular disease (PVD) and non-white race [15]. In a recent study of 17 000 Canadian patients, female gender and a higher number of co-morbidities were associated with lower rates of AVF creation [16]. The majority of studies reporting rates of AVF FTM have been conducted in the USA, which has a distinctly different healthcare system, higher rates of co-morbidities such as diabetes [17] and different ethnicity compared with European countries.
In 2006, Lok et al. [12] published a scoring system for stratifying AVF FTM risk based on clinical factors, designed to be used as an adjunct in planning vascular access for haemodialysis. The scoring system uses four clinical factors: age ≥65 years, race (white race associated with the lowest risk of FTM), presence of cardiovascular disease and presence of PVD to stratify patients into low, moderate, high and very high risks of FTM. The authors subsequently validated this risk model in a combined American–Canadian cohort of just over 400 patients. Our aim was to assess the application of this scoring system in a European population.
Materials and methods
Study setting and population
A prospective cohort study was designed that incorporated all patients undergoing native AVF creation between January 2009 and December 2014 in a single centre (Belfast City Hospital) who had a functional AVF outcome by March 2015. Functional outcomes were defined as follows:
Primary patency: Two-needle use of the AVF on haemodialysis for at least six consecutive sessions without further intervention following creation.
Primary assisted patency: AVF that required surgical or radiological intervention after initial creation before being used with two needles on haemodialysis for at least six consecutive sessions.
Functional patency: All AVFs used for two-needle dialysis. Functional patency was a combination of primary and primary assisted patency.
Failure to mature: Defined either by clinical examination or failure to sustain two-needle dialysis for at least six consecutive dialysis sessions.
Logistic regression was used to explore predictors of AVF FTM in the Belfast cohort. The Lok AVF FTM risk model [12] was then subsequently applied to our cohort. This risk model allows the formulation of a risk score to categorize FTM risk.
The risk score is calculated on the basis of the following:
- Age ≥65 years = 2 points
- Presence of coronary artery disease (CAD) (defined as coronary stenosis detected by angiography, history of myocardial infarction, previous coronary revascularization by angioplasty, stenting or cardiac bypass surgery) = 2.5 points
- Presence of PVD (defined as lower extremity revascularization, digit or extremity amputation, history of claudication and ischaemic extremity changes or gangrene) = 3 points
- White race = minus 3 points.
All patients receive a baseline score of 3 points.
The resultant overall score stratifies the patients into low risk (<2.0), moderate risk (2.0–3.0), high risk (3.1–6.9) and very high risk (7.0).
The Lok et al. derivation cohort [12] was comprised of 422 patients in Toronto (ON, Canada) who had received a first AVF between 1 January 1995 and 1 January 2004.
Data collection and study population
The Northern Ireland Vascular Access Database for Chronic Kidney Disease (ethics approval reference number 14/NI/1105) incorporates clinical and vascular access data on patients with advanced chronic kidney disease. Recorded patient characteristics included gender, age, race, primary renal disease, type of vascular access, anticoagulation use and co-morbidities including diabetes, CAD and PVD. The database was interrogated for these clinical characteristics in patients who had a functional AVF outcome by 1 March 2015. The ethics committee waived the need for individual patient consent. Each patient was only included once using outcome data on the first AVF created during January 2009–December 2014.
With regard to our vascular access creation strategy, our previous practice was to start distally in the non-dominant arm and then proceed proximally; i.e. a radiocephalic (wrist or proximal) AVF is attempted first, followed by a brachiocephalic AVF and then lastly a brachiobasilic AVF, subsequently moving to the dominant arm in order to preserve future vascular access options. Snuff-box AVFs and AVFs involving either the ulnar artery or forearm basilic vein were not created as standard practice in our centre. Since 2011, there has been increasing application of ultrasound assessment to help guide the choice of AVF creation site with minimum vessel diameters of 2 mm.
Following anastamosis, appropriate venous dilation must occur if an AVF is to mature successfully. During manipulation of the vein, it may spasm. Our surgical practice is to minimize handling of the blood vessel and to flush the outflow vein with a heparinized saline solution. Vasodilating agents are not used in our centre. Regarding the cannulation of new AVFs, it is our practice to carry out single-needle double-pump dialysis for the first three dialysis sessions and then subsequently establish all of our patients on two-needle dialysis.
Statistical analysis
The following clinical variables were identified for investigation regarding AVF FTM outcome prior to data collection: age, gender, renal replacement therapy status at AVF creation, diabetic status, CAD, PVD, anticoagulation use, previous AVF and location of AVF creation (upper-arm versus lower-arm site). Statistical analysis was performed using SPSS version 22 (IBM, Armonk, NY, USA).
Logistic regression was used to identify variables in the Belfast cohort that contributed significantly to predicting AVF FTM, as it disentangles the effects of correlated explanatory variables and takes account of confounders. The backward elimination approach was subsequently used to select the best set of independent variables.
The χ² test was used to compare baseline clinical characteristics between the Belfast cohort and the Lok model derivative cohort. As stated above, the Lok et al. prediction score [12] stratifies patients into low risk (<2.0), moderate risk (2.0–3.0), high risk (3.1–6.9) and very high risk (7.0) of AVF FTM. This score was subsequently used to stratify the Belfast cohort into the associated categories. Logistic regression analysis was subsequently used to assess for a significant difference between the low-risk category (set as a baseline categorical variable) FTM outcome and the other risk categories.
Results
A total of 688 patients were identified who had an AVF created by one of five consultant surgeons during this 6-year period. A functional AVF outcome was available for 538 patients on 1 March 2015. Of these, five patients had early technical failure and eight patients had their AVF ligated due to vascular steal before use. These 13 patients were excluded from the FTM analysis, leaving 525 patients that had a functional AVF outcome by 1 March 2015.
A total of 309 (59%) patients achieved functional AVF patency (primary patency n = 261, primary assisted patency n = 48), while 216 patients (41%) had FTM. In this cohort, 78% (240/309) of patients achieved functional patency after the creation of a single AVF.
Of the 216 patients who had AVF FTM, 44% (95/216) had no further surgical vascular access attempts and 56% (121/216) went on to have a mean of two further attempts at AVF creation. A functionally patent AVF was achieved in 71% (86/121) of patients who had further attempted AVF creation after initial FTM. In our clinical practice, the decision to reattempt further AVF creation is influenced by age, co-morbidities, remaining vessel ultrasound mapping characteristics and the option of an alternative renal replacement modality such as the availability of a living kidney donor.
With regard to exploring the predictors of AVF FTM, results of logistic regression analysis of multiple clinical variables are shown in Table 1.
Table 1.
Logistic regression analysis of predictors of AVF FTM in the Belfast cohort
| Clinical characteristic (reference category) | Odds ratio | 95% CI | P-value |
|---|---|---|---|
| Female gender (male = 0, female = 1) | 2.14 | 1.43–3.19 | <0.001 |
| Age ≥65 years (age < 65 years = 0, age ≥65 = 1) | 0.95 | 0.65–1.40 | 0.81 |
| Lower-arm site (lower-arm AVF = 1, upper arm = 0) | 4.24 | 2.86–6.27 | <0.001 |
| CKD at AVF creation (CKD = 1, HD = 2) | 1.20 | 0.85–1.67 | 0.31 |
| Anticoagulation (yes = 1, no = 0) | 1.02 | 0.68–1.56 | 0.92 |
| Co-morbidities | |||
| Diabetes mellitus (yes = 1, no = 0) | 0.93 | 0.62–1.40 | 0.74 |
| PVD (yes = 1, no = 0) | 1.55 | 0.84–2.86 | 0.16 |
| Ischaemic heart disease (yes = 1, no = 0) | 1.32 | 0.84–2.06 | 0.23 |
Backward elimination revealed that female gender [P < 0.001, odds ratio (OR) 2.04 (CI 1.37–3.02)] and lower-arm AVF [P < 0.001, OR 4.07 (CI 2.77–5.92)] were associated with AVF FTM in the Belfast cohort. Age, including age >80 years, was not associated with increased risk of FTM [P = 0.63, OR 1.12 (CI 0.69–1.83)].
Following these findings, subgroup analysis of the 186 female patients was subsequently carried out for the purposes of hypothesis generation. In the female cohort, only a lower-arm AVF was associated with a 4-fold risk of FTM [P < 0.001, OR 3.86 (CI 2.08–7.14)].
The Lok et al. AVF FTM risk prediction model [12] was then applied to the Belfast cohort. The clinical characteristics of the Belfast AVF cohort in comparison with the Lok et al. derivation cohort are shown in Table 2. In contrast to the Toronto cohort, the Belfast AVF cohort had higher proportions of older, white and diabetic patients.
Table 2.
Comparison of clinical characteristics in the Lok et al. [12] derivation model AVF cohort and Belfast AVF cohort
| Clinical characteristics | Original Lok cohort (n = 422) | Belfast cohort (n = 525) | P-value |
|---|---|---|---|
| Mean age, SD (range) | 58 years, 17.5 (17–90) | 64 years, 15 (14–93) | <0.001 |
| Age ≥65 years | 184 (44%) | 288 (55%) | <0.001 |
| Female gender | 136 (32%) | 186 (35%) | 0.301 |
| Race, white | 278 (65.8%) | 517 (98. 5%) | <0.001 |
| Cause of ESRD | |||
| Diabetes | 104 (24.6%) | 137 (26.1%) | 0.653 |
| Hypertension | 102 (24.2%) | 41 (7.8%) | <0.001 |
| Glomerulonephritis | 111 (26.3%) | 85 (16.2%) | <0.001 |
| Co-morbidities | |||
| Diabetes mellitus | 120 (28%) | 193 (37%) | 0.007 |
| CAD | 136 (32%) | 155 (30%) | 0.370 |
| PVD | 35 (8%) | 57 (11%) | 0.186 |
| Anticoagulation use | 260 (50%) | Not reported | |
| Pre-dialysis at creation | 194 (46%) | 277 (53%) | 0.038 |
| AVF type | |||
| Lower arm | 256 (60.7%) | 267 (51%) | 0.001 |
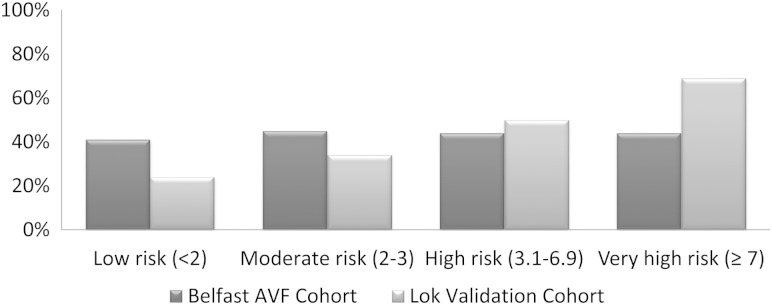
Following stratification of the Belfast cohort into the risk categories of the Lok et al. AVF FTM prediction mode [12], the proportion of patients with AVF FTM was 41% in the low-risk group, 45% in the moderate-risk group and 44% in the high- and very-high-risk groups (the predicted FTM rates for each of these categories based on the model were 25, 35, 50 and 70%, respectively). These differences in rates of FTM are illustrated in Figure 1.
Fig. 1.
Observed clinical FTM outcomes in the Belfast AVF cohort versus the predicted outcomes for this cohort using the Lok et al. risk model [12].
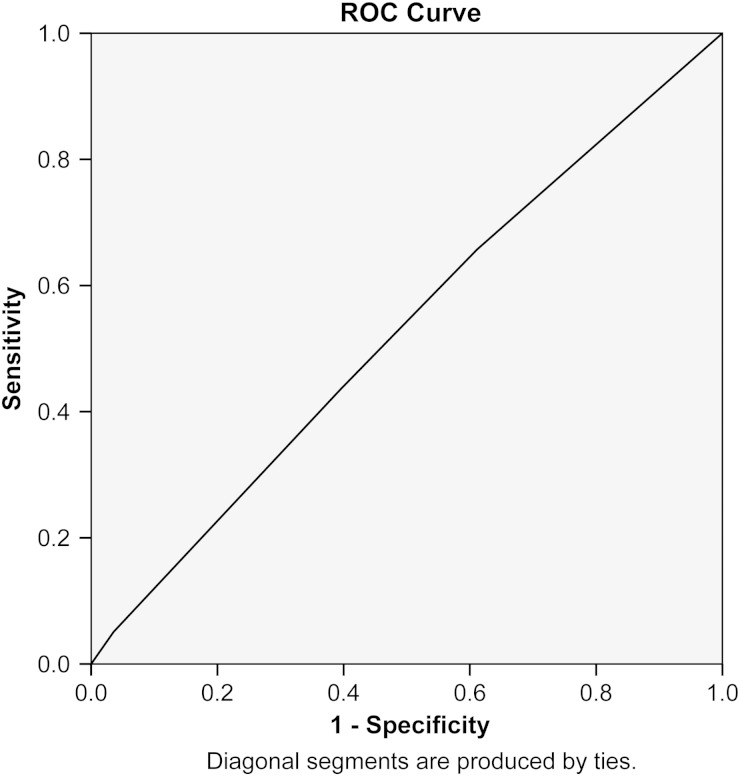
Using the Lok et al. risk as a categorical variable with the low-risk group representing the baseline, logistic regression analysis for predictors of AVF FTM showed no difference between FTM outcomes in the moderate- [P = 0.28, OR 0.62 (CI 0.26–1.49)], high- [P = 0.52, OR 0.75 (CI 0.31–1.81)] and very-high-risk categories [P = 0.45, OR 0.72 (CI 0.29–1.9)] compared with the low-risk category. Using receiver operator characteristic (ROC) analysis to investigate the ability of the model to predict FTM in this cohort revealed an area under the curve (C statistic) of 0.53 (P = 0.23, CI 0.48–0.58) (Figure 2). Thus, in our cohort of AVF patients, the Lok et al. risk model [12] did not discriminate between FTM outcomes.
Fig. 2.
Receiver operating analysis curve of the Lok et al. model [12] as a predictor of AVF FTM (area under the curve 0.53, P = 0.27, standard error 0.03, 95% CI 0.49–0.58).
Discussion
The lowest mortality rates in haemodialysis patients have been associated with AVF use and the highest mortality associated with CVC use for vascular access [3]. High rates of FTM are a major limiting factor to achieving optimal rates of incident and prevalent AVF use. The ability to stratify patients into AVF FTM risk categories is important in order to maximize patient outcomes and efficiently utilize limited healthcare resources. Our AVF patency rate is similar to other studies [13, 18].
Multiple studies have investigated clinical predictors of FTM with no general consensus [15, 19–21]. In our population of 525 patients, female gender and lower-arm AVF site were the only predictors of AVF FTM.
Female gender has been associated with increased risk of AVF FTM in several studies [15, 16, 22–24]. The reason for this remains unclear. In our cohort, there was no correlation between co-morbidities and increased risk of FTM in the female subgroup. Historically, the higher risk of FTM in females was attributed to smaller blood vessel diameter compared with males. However, despite female blood vessels being smaller in calibre than the equivalents in males, arterial diameters in females have been shown to be larger than the recommended minimum diameter of 2.0 mm [25] with similar venous diameters [26–28].
Following AVF creation, the feeding artery is required to accommodate a significantly increased blood flow; thus, arterial distensibility is likely to play a vital role in AVF maturation potential [29]. In a study of preoperative ultrasound assessment of radial artery characteristics prior to AVF creation, Lockhart et al. [26] found that female patients undergoing dialysis had a marked inability to increase their preoperative peak systolic flow following a clenched fist manoeuvre. This feature was associated with AVF FTM. A prospective European study of 122 patients by Jemkov [30] suggested that female patients require a longer period to attain AVF maturation despite having better endothelial function than men and recommended earlier AVF creation in this cohort of patients.
As found in our study, lower-arm AVFs are well known to have higher rates of FTM compared with upper-arm AVFs [23, 31]. The findings of higher AVF FTM in female patients and in lower-arm AVFs suggest that these patients may benefit from additional routine preoperative workup such as ultrasound mapping to identify the best blood vessels prior to attempted vascular access creation.
Age has been associated with increased AVF FTM in several studies [12, 15, 32]. Northern Ireland has one of the oldest cohorts of dialysis patients in the UK; in 2013, the median age of incident dialysis patients was 67 years [33], so Belfast is well placed to explore the role of age in AVF outcomes. It is interesting that even in patients >80 years, age was not associated with AVF FTM in our cohort, an observation that has been noted in other populations [34, 35].
The Lok et al. risk prediction model [12] for FTM is the only existing model for predicting AVF FTM based on purely clinical factors. Like us, Lilly et al. [15] found this model to be of limited value in predicting incident AVF use in an American cohort of haemodialysis patients. One of the reasons the model is not predictive in the Belfast cohort may be the differences in race composition; the Toronto cohort was 65.8% white and the Belfast cohort was 98.5% white. The predominantly white racial composition of the Belfast cohort is similar to the majority of European populations, which could suggest that the Lok et al. [12] model may be of limited value for European populations. This requires further investigation.
In both the US and Belfast populations, it is difficult to disentangle the effects of variables such as preoperative ultrasound mapping assessment and nephrological and surgical preferences in clinical practice [15]. Increasingly in our centre, ultrasound mapping is used to identify the best blood vessels and vascular access creation is then tailored to the individual. Routine ultrasound mapping at a nephrologist-led (J.B.H.) vascular access clinic was introduced in August 2011. For the elderly (age ≥65 years) or patients deemed to need dialysis initiation in a short period of time, we try to use the ‘best option’ identified by ultrasound mapping (lower or upper arm) to create the optimum AVF. During January 2009–December 2014, five surgeons have created AVFs at the Belfast City Hospital. One surgeon preferred a distal AVF first followed by a proximal AVF approach, two surgeons performed ultrasound measurements themselves, while the remaining two surgeons created AVFs on the basis of recommendations from the vascular access clinic findings (149/525 patients in this study). These differences and changes in practice are not readily accounted for in analyses, but are likely to have influenced AVF outcomes.
However, our results do show that positive AVF outcomes can be obtained in patients determined to be at high risk of AVF FTM based on age and co-morbidities; we would recommend that these variables alone should not be used to exclude patients from AVF creation.
Factors associated with AVF FTM are likely to vary from population to population. It is important to investigate local rates of AVF FTM and associated predictors of AVF patency in order to guide appropriate vascular access decision-making. For example, is the minimum preoperative vein diameter or radial artery volume flow in a 75-year-old female patient with diabetes the same as a 75-year-old female without diabetes? Information obtained from further studies should facilitate the creation of regionally applicable predictive models encompassing a combination of clinical and ultrasound parameters to help stratify patients into relevant categories of risk for AVF FTM.
Region-specific studies are required to guide appropriate vascular access management, thereby avoiding futile procedures and optimizing AVF access rates with the goal of enhancing survival in our dialysis patients.
Funding
A.M. is supported by a Northern Ireland Kidney Research Fund clinical research fellowship.
Conflict of interest statement
None declared.
Acknowledgements
We would like to acknowledge the hard work of our surgeons, without whom our patients would have no AVFs: J. Connolly, A. Pandey, M. Omar, T. Brown, J. McDaid and H. Magowan. We would also like to thank our vascular access nurses A. McCann and J. Wishart for their dedication and service to the unit and Chris Cardwell for his assistance with statistical analysis.
References
- 1.Steenkamp R, Rao A, Roderick P. UK Renal Registry 17th Annual Report: Chapter 5 Survival and Cause of Death in UK Adult Patients on Renal Replacement Therapy in 2013: National and Centre-specific Analyses. Nephron 2015; 129(Suppl. 1): 99–129 [DOI] [PubMed] [Google Scholar]
- 2.Ravani P, Palmer SC, Oliver MJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol 2013; 24: 465–473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang JC, Al-Jaishi AA, Na Y, et al. Association between vascular access type and patient mortality among elderly patients on hemodialysis in Canada. Hemodial Int 2014; 18: 616–624 [DOI] [PubMed] [Google Scholar]
- 4.Floege J, Gillespie IA, Kronenberg F, et al. Development and validation of a predictive mortality risk score from a European hemodialysis cohort. Kidney Int 2015; 87: 996–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drew DA, Lok CE, Cohen JT, et al. Vascular access choice in incident hemodialysis patients: a decision analysis. J Am Soc Nephrol 2015; 26: 183–191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Malas MB, Canner JK, Hicks CW, et al. Trends in incident hemodialysis access and mortality. JAMA Surg 2015; 150: 441–448 [DOI] [PubMed] [Google Scholar]
- 7.Dember LM, Beck GJ, Allon M, et al. Effect of clopidogrel on early failure of arteriovenous fistulas for hemodialysis: a randomized controlled trial. JAMA 2008; 299: 2164–2171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Georgiadis GS, Charalampidis DG, Argyriou C, et al. The necessity for routine pre-operative ultrasound mapping before arteriovenous fistula creation: a meta-analysis. Eur J Vasc Endovasc Surg 2015; 49: 600–605 [DOI] [PubMed] [Google Scholar]
- 9.van der Veer SN, Ravani P, Coentrao L, et al. Barriers to adopting a fistula-first policy in Europe: an international survey among national experts. J Vasc Access 2015; 16: 113–119 [DOI] [PubMed] [Google Scholar]
- 10.Nica A, Lok CE, Harris J, et al. Understanding surgical preference and practice in hemodialysis vascular access creation. Semin Dial 2013; 26: 520–526 [DOI] [PubMed] [Google Scholar]
- 11.Xi W, MacNab J, Lok CE, et al. Who should be referred for a fistula? A survey of nephrologists. Nephrol Dial Transplant 2010; 25: 2644–2651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lok CE, Allon M, Moist L, et al. Risk equation determining unsuccessful cannulation events and failure to maturation in arteriovenous fistulas (REDUCE FTM I). J Am Soc Nephrol 2006; 17: 3204–3212 [DOI] [PubMed] [Google Scholar]
- 13.Bashar K, Zafar A, Elsheikh S, et al. Predictive parameters of arteriovenous fistula functional maturation in a population of patients with end-stage renal disease. PLoS One 2015; 10: e0119958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aitken E, Jackson A, Kong C, et al. Renal function, uraemia and early arteriovenous fistula failure. BMC Nephrol 2014; 15: 179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lilly MP, Lynch JR, Wish JB, et al. Prevalence of arteriovenous fistulas in incident hemodialysis patients: correlation with patient factors that may be associated with maturation failure. Am J Kidney Dis 2012; 59: 541–549 [DOI] [PubMed] [Google Scholar]
- 16.Al-Jaishi AA, Lok CE, Garg AX, et al. Vascular access creation before hemodialysis initiation and use: a population-based cohort study. Clin J Am Soc Nephrol 2015; 10: 418–427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US Renal Data System. United States Renal Data System 2014 Annual Data Report: Epidemiology of Kidney Disease in the United States. Bethesda, MD: US Renal Data System, 2014 [Google Scholar]
- 18.Huijbregts HJ, Bots ML, Wittens CH, et al. Hemodialysis arteriovenous fistula patency revisited: results of a prospective, multicenter initiative. Clin J Am Soc Nephrol 2008; 3: 714–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravani P, Brunori G, Mandolfo S, et al. Cardiovascular comorbidity and late referral impact arteriovenous fistula survival: a prospective multicenter study. J Am Soc Nephrol 2004; 15: 204–209 [DOI] [PubMed] [Google Scholar]
- 20.Wang W, Murphy B, Yilmaz S, et al. Comorbidities do not influence primary fistula success in incident hemodialysis patients: a prospective study. Clin J Am Soc Nephrol 2008; 3: 78–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stoumpos S, Stevens KK, Aitken E, et al. Predictors of sustained arteriovenous access use for haemodialysis. Am J Nephrol 2014; 39: 491–498 [DOI] [PubMed] [Google Scholar]
- 22.Miller CD, Robbin ML, Allon M. Gender differences in outcomes of arteriovenous fistulas in hemodialysis patients. Kidney Int 2003; 63: 346–352 [DOI] [PubMed] [Google Scholar]
- 23.Peterson WJ, Barker J, Allon M. Disparities in fistula maturation persist despite preoperative vascular mapping. Clin J Am Soc Nephrol 2008; 3: 437–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Miller PE, Tolwani A, Luscy CP, et al. Predictors of adequacy of arteriovenous fistulas in hemodialysis patients. Kidney Int 1999; 56: 275–280 [DOI] [PubMed] [Google Scholar]
- 25.Silva MB, Jr, Hobson RW, 2nd, Pappas PJ, et al. A strategy for increasing use of autogenous hemodialysis access procedures: impact of preoperative noninvasive evaluation. J Vasc Surg 1998; 27: 302–307; discussion 307–308 [DOI] [PubMed] [Google Scholar]
- 26.Lockhart ME, Robbin ML, Allon M. Preoperative sonographic radial artery evaluation and correlation with subsequent radiocephalic fistula outcome. J Ultrasound Med 2004; 23: 161–168; quiz 169–171 [DOI] [PubMed] [Google Scholar]
- 27.Saucy F, Haesler E, Haller C, et al. Is intra-operative blood flow predictive for early failure of radiocephalic arteriovenous fistula? Nephrol Dial Transplant 2010; 25: 862–867 [DOI] [PubMed] [Google Scholar]
- 28.Caplin N, Sedlacek M, Teodorescu V, et al. Venous access: women are equal. Am J Kidney Dis 2003; 41: 429–432 [DOI] [PubMed] [Google Scholar]
- 29.Kheda MF, Brenner LE, Patel MJ, et al. Influence of arterial elasticity and vessel dilatation on arteriovenous fistula maturation: a prospective cohort study. Nephrol Dial Transplant 2010; 25: 525–531 [DOI] [PubMed] [Google Scholar]
- 30.Jemcov TK. Morphologic and functional vessels characteristics assessed by ultrasonography for prediction of radiocephalic fistula maturation. J Vasc Access 2013; 14: 356–363 [DOI] [PubMed] [Google Scholar]
- 31.Al-Jaishi AA, Oliver MJ, Thomas SM, et al. Patency rates of the arteriovenous fistula for hemodialysis: a systematic review and meta-analysis. Am J Kidney Dis 2014; 63: 464–478 [DOI] [PubMed] [Google Scholar]
- 32.Hod T, Desilva RN, Patibandla BK, et al. Factors predicting failure of AV ‘fistula first’ policy in the elderly. Hemodial Int 2014; 18: 507–515 [DOI] [PubMed] [Google Scholar]
- 33.Gilg J, Rao A, Fogarty D. UK Renal Registry 16th Annual Report: Chapter 1 UK Renal Replacement Therapy Incidence in 2012: National and Centre-Specific Analyses. Nephron Clin Pract 2013; 125: 1–27 [DOI] [PubMed] [Google Scholar]
- 34.Weyde W, Letachowicz W, Kusztal M, et al. Outcome of autogenous fistula construction in hemodialyzed patients over 75 years of age. Blood Purif 2006; 24: 190–195 [DOI] [PubMed] [Google Scholar]
- 35.Watorek E, Golebiowski T, Kusztal M, et al. Creation of arteriovenous fistulae for hemodialysis in octogenarians. Hemodial Int 2014; 18: 113–117 [DOI] [PubMed] [Google Scholar]