Abstract
Background
Inflammation and serum albumin concentration are both important predictors of survival in patients treated with peritoneal dialysis (PD). Furthermore, systemic and local inflammatory mediators may induce structural and functional alterations in the peritoneal membrane, thus interfering with dialysis adequacy. PD adequacy is monitored primarily by indices of small solute clearance, such as Kt/V urea and weekly creatinine clearance (wCc). The aim of this study was to investigate the possible relationship between pro-inflammatory cytokines, such as interleukin-6 (IL-6) and interleukin-1β (IL-1β), and serum albumin and C-reactive protein (CRP). Moreover, the relationship between IL-6 and IL-1β and PD adequacy has been analysed.
Methods
We enrolled 46 stable PD patients undergoing maintenance PD for a minimum of 3 months. Plasma levels of serum albumin, high-sensitivity (hs)-CRP, IL-6 and IL-1β were measured in all patients. We used weekly Kt/V urea and wCc to monitor PD adequacy. Daily urine volume was measured in all patients.
Results
The median values of serum albumin, hs-CRP, IL-6 and IL-1β showed no significant differences between continuous ambulatory PD and automated PD patients. IL-6 levels showed a positive correlation with hs-CRP levels (P < 0.001) and a negative correlation with serum albumin concentration (P = 0.01). There was no statistically significant relationship between IL-1β and hs-CRP or serum albumin concentrations. Subsequently, PD patients were divided into two groups based on Kt/V urea value. PD patients with Kt/V ≤1.7 had significantly higher IL-6 levels compared with PD patients with Kt/V >1.7 (P = 0.015). No statistically significant relationship between IL-6 and wCc was observed. There was no significant difference in IL-1β levels between PD patients with Kt/V ≤1.7 and with Kt/V >1.7 [median (interquartile range) 0.82 (0.88–5.2) versus 1.82 (0.95–2.7)]. There was no significant difference in IL-6 and IL-1β levels in PD patients with and without residual diuresis (P = 0.32 and P = 0.77, respectively).
Conclusion
Our data suggest a possible relationship between serum IL-6 levels and serum albumin and hs-CRP in PD patients. Furthermore, IL-6 seems to be higher in patients with lower Kt/V, thus suggesting a possible use of this inflammatory biomarker in PD adequacy monitoring.
Keywords: cytokines, dialytic adequacy, inflammation, peritoneal dialysis, serum albumin
Introduction
Inflammation and serum albumin concentration are important predictors of survival in patients undergoing peritoneal dialysis (PD) for advanced renal failure [1].
In PD patients, there are two types of inflammation, systemic and local intraperitoneal inflammation. The reported prevalence of systemic inflammation measured using C-reactive protein (CRP) ranges between 12 and 65%, depending on the cut-off value used to define the level of inflammation [2, 3]. Increasing burden of inflammation measured using interleukin-6 (IL-6) with longer time on PD at both systemic and intraperitoneal levels has been documented as well [4, 5]. Causes of inflammation in PD range from traditional factors related to chronic kidney disease per se to those related to the dialytic treatment, such as peritoneal catheter and dialysis solutions [6]. All these factors contribute to the release of pro-inflammatory mediators, such as interleukin-1β (IL-1β), IL-6, tumour necrosis factor α (TNF-α) and interleukin-18 (IL-18) [6], which may induce structural modifications in the peritoneal membrane, thus leading to altered small solute clearance and ultrafiltration failure. PD adequacy is primarily monitored by indices of small solute clearance, such as Kt/V urea and weekly creatinine clearance (wCc) [7], but its relationship with inflammatory cytokines remains largely unknown.
Even though serum albumin is an acute-phase protein, it has been reported that its concentration may be independently reduced by inflammation [8]. Pro-inflammatory cytokines also trigger the acute-phase response in the liver [9], thus increasing serum concentration of CRP, fibrinogen and amyloid A protein. As part of the acute-phase response, there is also a reduction in synthesis and an increase in degradation of albumin that results in hypoalbuminaemia [10].
The aim of this study was to investigate the possible relationship between pro-inflammatory cytokines, such as IL-6 and IL-1β, and serum albumin and CRP. Moreover, the relationship between IL-6 and IL-1β and PD adequacy has been analysed.
Materials and methods
Subjects
This cross-sectional study was conducted over a 2-month period (1 April 2013–31 May 2013) in the Peritoneal Dialysis Unit of San Bortolo Hospital, Vicenza.
A total of 70 PD patients, who were older than 18 years and who were on PD maintenance for a minimum of 3 months, were screened and medical histories were reviewed for inclusion into the study. Forty-six PD patients in a stable condition and free from intercurrent illness and infections for at least the last 3 months were finally enrolled into the study. Twenty-four PD patients with serious medical comorbidities, such as active infections, autoimmune disease, malignancies, unstable angina, end-stage cardiac insufficiency, pulmonary or hepatic disease, were excluded.
None of the patients included in the study was treated with immunosuppressive drugs, but most of them were treated with erythropoietin and intravenous iron. Clinical characteristics, laboratory data and dialysis-related parameters were recorded for all patients. Weekly Kt/V urea and wCc were used as estimates of PD adequacy. Weekly Kt/V urea and wCC were calculated based on 24-h urine performed prior to the scheduled visit to the PD unit. PD patients were divided into two groups based on Kt/V urea value: 1.7 was the cut-off value as recommended by K/DOQI guidelines [7].
Sample collection and laboratory parameters
Blood samples were collected from all 46 patients into EDTA-containing tubes and processed within 1 h after venipuncture. Samples were subsequently centrifuged for 7 min at 1600 g.
Blood urea nitrogen, serum creatinine (Cr), albumin, high-sensitivity C-reactive protein (hs-CRP) and other biochemistry parameters were measured by standard laboratory techniques with an automatic analyser (Dimension Vista, Siemens Healthcare, Tarrytown, NY, USA). White blood cells, haemoglobin, and haematocrit were measured by the automated haematology analysers XN 9000 (SYSMEX, KOBE, Japan). Erythrocyte sedimentation rate was measured by the Vesmatic Cube 200 (Diesse Diagnostica Senese, Siena, Italy).
Cytokines enzyme-linked immunosorbent assay
Quantitative determination of cytokines (IL-1β and IL-6) in plasma was performed by Human Instant Enzyme-Linked Immunosorbent Assay (ELISA) kit (eBioscience, San Diego, CA, USA) according to the manufacturer's instructions. Optical density was read by using a VICTORX4 Multilabel Plate Reader (PerkinElmer Life Sciences, Waltham, MA, USA) at 450 nm. The concentration values for these molecules were calculated from standard curves. All tests were performed in triplicate.
Statistical analysis
Statistical analysis was performed using the STATA Software package. Categorical variables were expressed as percentages; continuous variables were expressed as means ± standard deviation (parametric variables) or median and interquartile range (IQR) (nonparametric variables). The Mann–Whitney U-test was used for comparison of two groups, as appropriate. Correlation coefficients were calculated with the Spearman rank correlation coefficient test. A P-value of <0.05 was considered statistically significant.
Results
Subjects' baseline characteristics
A total of 46 PD patients (25 males and 21 females; mean age 61.5 ± 16.4 years, median time on PD 26.4 months; IQR 13.3–51.4 months) were enrolled in this study. End-stage renal failure in the study population was attributed to diabetic nephropathy (12/46, 26.1% of patients), glomerulosclerosis (11/46, 23.9%), nephroangiosclerosis (9/46, 19.6%), autosomic polycystic kidney disease (3/46, 6.5%), vesico-ureteral reflux (1/46, 2.2%), pseudoxanthoma elasticum (1/46, 2.2%) or unknown causes (9/46, 19.6%). Furthermore, 31/46 patients were treated with continuous ambulatory PD (CAPD) and 15/46 with automated PD (APD). The average length of PD treatment was 26.4 months and the range was (minimum–maximum): 3.6–132.9 months. Out of the 46 patients, 25 did not have any episode of peritonitis, 12/46 patients had only one episode of peritonitis, 5/46 patients had two episodes of peritonitis, 1/46 patients had three episodes of peritonitis and 3/46 had four episodes of peritonitis. Out of 46 patients 31 had a residual diuresis. The median daily urine volume was 550 mL (IQR 0–1100, minimum–maximum 0–2000 mL).
The clinical, laboratory and dialysis-related parameters of all 46 patients are summarized in Table 1.
Table 1.
Baseline characteristics of 46 PD patients
| Male/female | 25 (54.3%) |
| Age, years | 63 ± 16.4 |
| CAPD | 31/46 (67.4%) |
| APD | 15/46 (32.6%) |
| PD time, months | 26.4 (13.3–51.4) |
| Residual diuresis | 31/46 (67.4%) |
| Total wCc (l/week/1.73 m2) | 72.7 (53–102.6) |
| Total weekly Kt/V urea | 1.95 (1.63–2.21) |
| Kt/V ≤1.7 | 14/46 (30.4%) |
| Kt/V >1.7 | 32/46 (69.6%) |
| PET ≤0.6 | 2/46 (4.3%) |
| 0.6 < PET ≤ 0.8 | 34/46 (73.9%) |
| PET >0.8 | 10/46 (21.7%) |
PET, peritoneal equilibration test.
Laboratory parameters and cytokines concentration in PD patients
Pro-inflammatory cytokine levels (IL-1β and IL-6) were measured by ELISA in plasma of all 46 PD patients at recruiting time. Laboratory parameters and cytokines levels are reported in Table 2. These data did not follow a Gaussian distribution. No outliers or extreme values were found. The median values of serum albumin, hs-CRP, IL-1β and IL-6 showed no significant differences between CAPD and APD patients. IL-6 levels showed a positive correlation with hs-CRP (Spearman rank = 0.52, P < 0.001) (Figure 1). IL-6 correlated negatively with serum albumin (Spearman rank = −0.35, P = 0.01) (Figure 2) and with Kt/V values (Spearman rank = −0.34, P = 0.04). No statistically significant relationship was observed between IL-1β and hs-CRP or serum albumin.
Table 2.
Laboratory parameters and cytokines levels in all 46 PD patients
| Laboratory parameters | Median (IQR) |
|---|---|
| Haemoglobin, g/dL | 11.9 (11.4–12.9) |
| Lymphocyte count, ×109/L | 6.9 (5.5–8.15) |
| hs-CRP, mg/dL | 0.33 (0.28–0.81) |
| Albumin, g/dL | 3.0 (2.7–3.2) |
| IL-6, pg/mL | 21.4 (9.4–61.9) |
| IL-1β, pg/mL | 1.82 (0.87–2.92) |
Fig. 1.
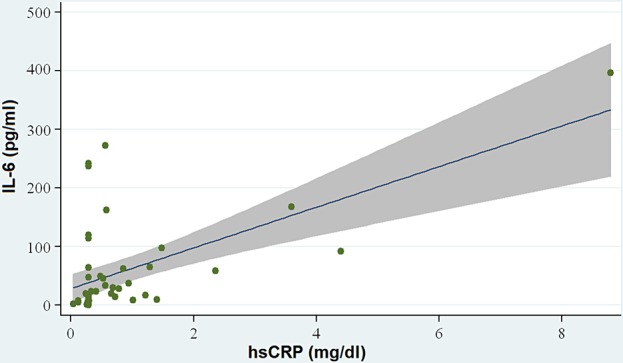
Correlation between IL-6 and CRP: IL-6 levels showed a positive correlation with CRP (P < 0.001).
Fig. 2.
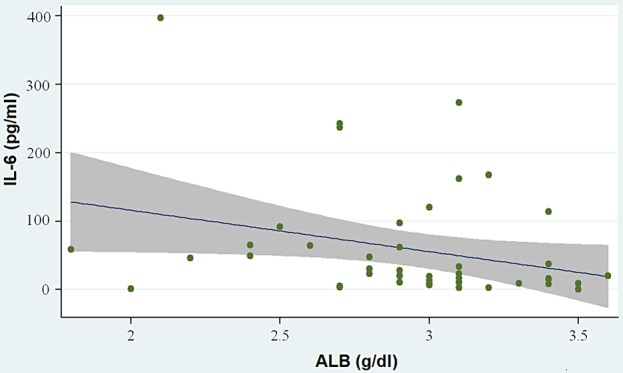
Correlation between IL-6 and serum albumin (ALB): IL-6 correlated negatively with serum albumin (P = 0.01).
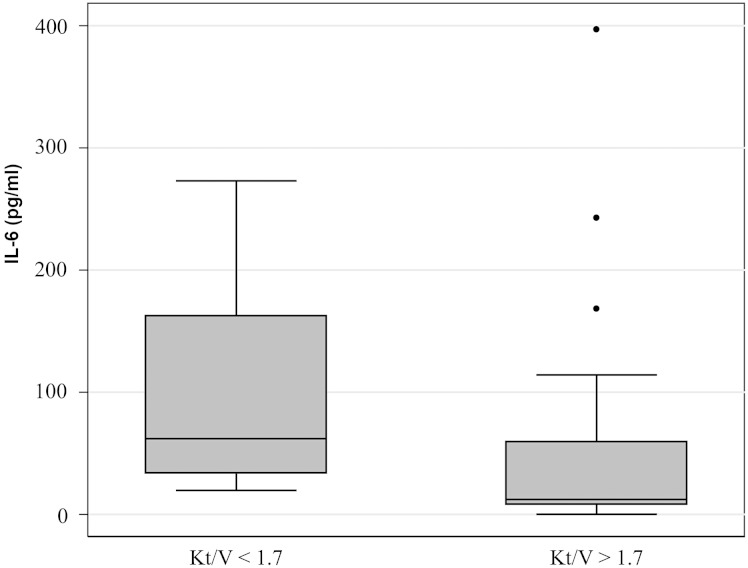
PD patients with Kt/V ≤1.7 had significantly higher IL-6 compared with PD patients with Kt/V >1.7 (P = 0.015) (Figure 3). The median value of IL-6 in PD patients with Kt/V ≤1.7 was 62 pg/mL (IQR 33–162). The median value of IL-6 in PD patients with Kt/V >1.7 was 12 pg/mL (IQR 8–59). Nevertheless, the association between IL-6 and wCc did not reach statistical significance.
Fig. 3.
PD patients with Kt/V ≤1.7 had significantly higher IL-6 compared with PD patients with Kt/V >1.7 (median 62 pg/mL, IQR 33–162 versus 12 pg/mL, IQR 8–59).
PD patients with Kt/V ≤1.7 showed a median urine volume of 550 mL (IQR 0–700, minimum–maximum 0–1000 mL) versus 575 (IQR 0–778, minimum–maximum 0–2000 mL) in PD patients with Kt/V >1.7. There was no difference in IL-6 levels in PD patients with and without residual diuresis (20 pg/mL, 9–49 versus 23 pg/mL, 14–65; P = 0.32). Furthermore, the association between IL-6 and daily urine volume value did not reach statistical significance (P = 0.33).
There was no difference in IL-1β levels in PD patients with Kt/V ≤1.7 and with Kt/V >1.7 (P = 0.89). PD patients with Kt/V ≤1.7 showed a median IL-1β levels of 0.82 pg/mL (IQR 0.88–5.2) versus 1.82 pg/mL (IQR 0.95–2.7) in PD patients with Kt/V >1.7. There was no difference in IL-1β levels in PD patients with and without residual diuresis (2.4 pg/mL, IQR 1.4–3.1, minimum–maximum 0.52–21.8 versus 0.7 pg/mL, 0.6–1.4, minimum–maximum 0.25–26.4; P = 0.08)
Discussion
Inflammation is a powerful predictor of adverse cardiovascular events and mortality in PD patients. Different factors including bioincompatibility of conventional glucose-based PD solutions, peritonitis, exit site infections, accumulation of uraemic toxins, malnutrition and adiposity seem to play a pivotal role in its pathogenesis. The consequent release of pro-inflammatory mediators such as IL-1β, IL-6, TNF-α and IL-18 [6] may interfere with peritoneal adequacy by inducing both structural and functional in the peritoneal membrane.
The concept of dialysis adequacy was introduced to evaluate the effect of renal replacement therapy on clinical outcomes in patients with end-stage renal disease. Adequate dialysis is defined as such amount of dialysis therapy that is sufficient to protect from increased mortality and morbidity [7]. Dialysis adequacy is judged by clinical parameters (patient well-being and lack of uraemic symptoms, good nutrition, appropriate blood pressure control, stable body weight, normal fluid balance, and appropriate growth rate and psychosocial development) and laboratory data (appropriate urea, creatinine, electrolyte, albumin and haemoglobin levels, and lack of metabolic acidosis) [11].
In our study, IL-6 showed a positive correlation with hs-CRP and a negative correlation with serum albumin.
IL-6 is one of the most studied inflammatory biomarkers in PD patients. In particular, available data suggest that IL-6 and its soluble receptor (sIL-6R) are central regulators of both innate and acquired inflammatory processes. IL-6 activates macrophages and lymphocytes, induces expression of adhesion molecules by endothelial cells, promotes a prothrombothic state and increases endothelial dysfunction [2]. Moreover, IL-6 is secreted in large quantities by peritoneal mesothelial cells in response to inflammatory stimuli and modulated by exposure to PD solutions [12].
Together with IL-1β and TNF-α, IL-6 is known to induce the acute-phase response in the liver [9], thus increasing serum concentration of CRP, fibrinogen and amyloid A protein. As part of the acute-phase response, there is also a reduction in synthesis and an increase in degradation of albumin that results in hypoalbuminaemia [10]. The importance of the acute-phase response as a cause of hypoalbuminaemia has been demonstrated in several studies where a negative correlation between serum albumin and CRP and fibrinogen has been found [13, 14]. We can hypothesize that in the presence of high levels of IL-6 in PD patients, low serum albumin concentration may result from inflammatory mechanisms triggered by factors related both to chronic kidney disease and dialytic treatment. Conversely, in the presence of normal level of IL-6, in particular if Kt/V is low, hypoalbuminaemia may be caused by underdialysis and consequent reduced nutritional intake. This concept highlights the rationale for reconsidering serum albumin also as a marker of inflammation and not only as a marker of nutrition. Our hypothesis is supported by a recent review performed by Friedman and Fadem who reported that the relationship between serum albumin and malnutrition is not always reliable [15]. Furthermore, Shioya et al. have recently demonstrated that hypoalbuminaemia in PD patients may be induced by both the increased peritoneal permeability and systemic inflammation, and intraperitoneal inflammation might contribute to the development of these conditions [16].
In our study, patients with lower Kt/V had significantly higher IL-6 concentrations, independently of residual diuresis, which was similar between patients with Kt/V ≤ 1.7 and patients with Kt/V >1.7. There was, indeed, no significant difference in IL-6 levels in PD patients with and without residual diuresis.
The role of the residual renal function (RRF) in the setting of cytokines clearance in PD patients is still debated. Likely, patients with Kt/V ≤ 1.7 have less RRF. RRF has a beneficial effect on survival in PD patients [17, 18], due to a better fluid balance, a superior phosphorus control and a prolonged preservation of renal endocrine functions [19, 20]. Furthermore, an association between lowered RRF and higher systemic inflammatory burden in dialysis patients has already been reported [21, 22]. Indeed, the level of inflammatory cytokines progressively increases with worsening renal function [23]. Anuric PD patients show the greatest inflammatory response as measured either by CRP [11] or by soluble vascular cell adhesion molecule 1 [24]. Loss of RRF has been suggested to increase oxidative stress, a response that may lead to monocyte activation and cytokine production [25]. Anyway, it remains uncertain whether these conditions are primarily a result of an impaired renal clearance of inflammatory cytokines, direct stimulation of cytokine release by uraemic milieu or simply a consequence of an adverse effect of inflammation on RRF.
Moreover, the relationship between RRF and inflammation becomes less clear once PD is started due to specific factors related to the treatment, such as peritonitis and PD solutions exposure, responsible for systemic inflammatory cytokines production independent of the RRF decline.
Since in our study patients with lower Kt/V showed higher levels of IL-6, independently of residual diuresis, we can hypothesize that IL-6 concentration may be related to PD adequacy. Our data are supported by previous studies. The GLOBAL fluid observational study, which included 959 PD patients, did not report any significant association between patients' residual urine volume and serum IL-6 concentrations in their prevalent or incident cohort [26]. Similarly, a substudy of the balANZ trial did not observe any significant association between the loss of RRF and serum IL-6 levels over a follow-up period of 24 months in 175 incident PD patients [27]. According to the results of these studies, the higher levels of IL-6 observed in patients with Kt/V ≤ 1.7 may indicate a reduced peritoneal adequacy, which might be in part due to the higher level of inflammation in these patients. Repeated exposures to conventional PD solutions and peritonitis episodes contribute to peritoneal injury, which is responsible for local inflammation with resultant adverse functional outcomes, such as higher peritoneal solute transport rate [28, 29]. Indeed, dialysate IL-6 concentration has been identified as the most reliable predictor of peritoneal solute transport rate by different single centre studies and has been corroborated by the recent analysis of the GLOBAL study [4, 26].
In our study, no statistically significant relationship between IL-1β and CRP, serum albumin, diuresis and PD adequacy was observed.
Nevertheless, our study has some limitations. First, it is a small, monocentric study, reflecting the general difficulty in performing clinical studies on PD patients while excluding patients with confounding variables or aiming to study groups with similar demographics characteristics. Second, we did not collect peritoneal effluent fluid and we did not evaluate the cytokine levels in the effluent.
Conclusions
To our knowledge, this is the first study investigating the possible relationship between pro-inflammatory cytokines, IL-1β and IL-6, and CRP, albumin, diuresis and PD adequacy. Our data suggest a possible relationship between serum IL-6 levels and serum albumin and hs-CRP in PD patients. Furthermore, IL-6 seems to be higher in patients with lower Kt/V, thus suggesting a possible use of this inflammatory biomarker in PD adequacy monitoring. Conversely, no significant relationship was found between IL-1β and CRP, albumin and PD adequacy.
Our preliminary results can be considered as hypothesis generating, allowing further exploration of the novel role of IL-6 in PD patients. Further studies are needed to evaluate the utility and the potentiality of IL-6 as an alternative circulating biomarker for PD adequacy monitoring.
Conflict of interest statement
None declared.
References
- 1.Mehrotra R, Duong U, Jiwakanon S, et al. Serum albumin as a predictor of mortality in peritoneal dialysis: comparisons with hemodialysis. Am J Kidney Dis 2011; 58: 418–428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang AY. Consequences of chronic inflammation in peritoneal dialysis. Semin Nephrol 2011; 31: 159–171 [DOI] [PubMed] [Google Scholar]
- 3.Wang AY. Prognostic value of C-reactive protein for heart disease in dialysis patients. Curr Opin Investig Drugs 2005; 6: 879–886 [PubMed] [Google Scholar]
- 4.Pecoits-Filho R, Carvalho MJ, Stenvinkel P, et al. Systemic and intraperitoneal interleukin-6 system during the first year of peritoneal dialysis. Perit Dial Int 2006; 26: 53–63 [PubMed] [Google Scholar]
- 5.Cho Y, Johnson DW, Vesey DA, et al. Dialysate interleukin-6 predicts increasing peritoneal solute transport rate in incident peritoneal dialysis patients. BMC Nephrol 2014; 15: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baroni G, Schuinski A, de Moraes TP, et al. Inflammation and the peritoneal membrane: causes and impact on structure and function during peritoneal dialysis. Mediators Inflamm 2012; 2012: 912595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinical Practice Guidelines and Recommendations on Peritoneal Dialysis Adequacy 2011. Perit Dial Int 2011; 31: 218–239 [DOI] [PubMed] [Google Scholar]
- 8.KDOQI Clinical Practice Guideline for Nutrition in Children with CKD: 2008 update. Executive summary. KDOQI Work Group. Am J Kidney Dis 2009; 53(3 Suppl 2): S11–S104 [DOI] [PubMed] [Google Scholar]
- 9.Ramadori G, Van Damme J, Rieder H, et al. Interleukin 6, the third mediator of acute-phase reaction, modulates hepatic protein synthesis in human and mouse. Comparison with interleukin 1 beta and tumor necrosis factor-alpha. Eur J Immunol 1988; 18: 1259–1264 [DOI] [PubMed] [Google Scholar]
- 10.Doweiko JP, Nompleggi DJ. The role of albumin in human physiology and pathophysiology, Part III: albumin and disease states. JPEN J Parenter Enteral Nutr 1991; 15: 476–483 [DOI] [PubMed] [Google Scholar]
- 11.Wang AY, Woo J, Lam CW, et al. Is a single time point C-reactive protein predictive of outcome in peritoneal dialysis patients? J Am Soc Nephrol 2003; 14: 1871–1879 [DOI] [PubMed] [Google Scholar]
- 12.Witowski J, Jorres A, Coles GA, et al. Superinduction of IL-6 synthesis in human peritoneal mesothelial cells is related to the induction and stabilization of IL-6 mRNA. Kidney Int 1996; 50: 1212–1223 [DOI] [PubMed] [Google Scholar]
- 13.Kaysen GA, Rathore V, Shearer GC, et al. Mechanisms of hypoalbuminemia in hemodialysis patients. Kidney Int 1995; 48: 510–516 [DOI] [PubMed] [Google Scholar]
- 14.Kaysen GA, Stevenson FT, Depner TA. Determinants of albumin concentration in hemodialysis patients. Am J Kidney Dis 1997; 29: 658–668 [DOI] [PubMed] [Google Scholar]
- 15.Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol 2010; 21: 223–230 [DOI] [PubMed] [Google Scholar]
- 16.Shioya M, Yoshida T, Kasai K, et al. Inflammatory factors for hypoalbuminemia in Japanese peritoneal dialysis patients. Nephrology (Carlton) 2013; 18: 539–544 [DOI] [PubMed] [Google Scholar]
- 17.Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol 2001; 12: 2158–2162 [DOI] [PubMed] [Google Scholar]
- 18.Paniagua R, Amato D, Vonesh E, et al. Effects of increased peritoneal clearances on mortality rates in peritoneal dialysis: ADEMEX, a prospective, randomized, controlled trial. J Am Soc Nephrol 2002; 13: 1307–1320 [DOI] [PubMed] [Google Scholar]
- 19.Ates K, Nergizoglu G, Keven K, et al. Effect of fluid and sodium removal on mortality in peritoneal dialysis patients. Kidney Int 2001; 60: 767–776 [DOI] [PubMed] [Google Scholar]
- 20.Burkart JM. The ADEMEX study and PD adequacy. Blood Purif 2003; 21: 37–41 [DOI] [PubMed] [Google Scholar]
- 21.Chung SH, Heimburger O, Stenvinkel P, et al. Association between inflammation and changes in residual renal function and peritoneal transport rate during the first year of dialysis. Nephrol Dial Transplant 2001; 16: 2240–2245 [DOI] [PubMed] [Google Scholar]
- 22.Borazan A, Ustun H, Ustundag Y, et al. The effects of peritoneal dialysis and hemodialysis on serum tumor necrosis factor-alpha, interleukin-6, interleukin-10 and C-reactive-protein levels. Mediators Inflamm 2004; 13: 201–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Descamps-Latscha B, Herbelin A, Nguyen AT, et al. Balance between IL-1 beta, TNF-alpha, and their specific inhibitors in chronic renal failure and maintenance dialysis. Relationships with activation markers of T cells, B cells, and monocytes. J Immunol 1995; 154: 882–892 [PubMed] [Google Scholar]
- 24.Wang AY, Lam CW, Wang M, et al. Circulating soluble vascular cell adhesion molecule 1: relationships with residual renal function, cardiac hypertrophy, and outcome of peritoneal dialysis patients. Am J Kidney Dis 2005; 45: 715–729 [DOI] [PubMed] [Google Scholar]
- 25.Witko-Sarsat V, Friedlander M, Nguyen Khoa T, et al. Advanced oxidation protein products as novel mediators of inflammation and monocyte activation in chronic renal failure. J Immunol 1998; 161: 2524–2532 [PubMed] [Google Scholar]
- 26.Lambie M, Chess J, Donovan KL, et al. Independent effects of systemic and peritoneal inflammation on peritoneal dialysis survival. J Am Soc Nephrol 2013; 24: 2071–2080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho Y, Johnson DW, Vesey DA, et al. Baseline serum interleukin-6 predicts cardiovascular events in incident peritoneal dialysis patients. Perit Dial Int 2015; 35: 35–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davies SJ, Phillips L, Russell GI. Peritoneal solute transport predicts survival on CAPD independently of residual renal function. Nephrol Dial Transplant 1998; 13: 962–968 [DOI] [PubMed] [Google Scholar]
- 29.Rumpsfeld M, McDonald SP, Johnson DW. Higher peritoneal transport status is associated with higher mortality and technique failure in the Australian and New Zealand peritoneal dialysis patient populations. J Am Soc Nephrol 2006; 17: 271–278 [DOI] [PubMed] [Google Scholar]