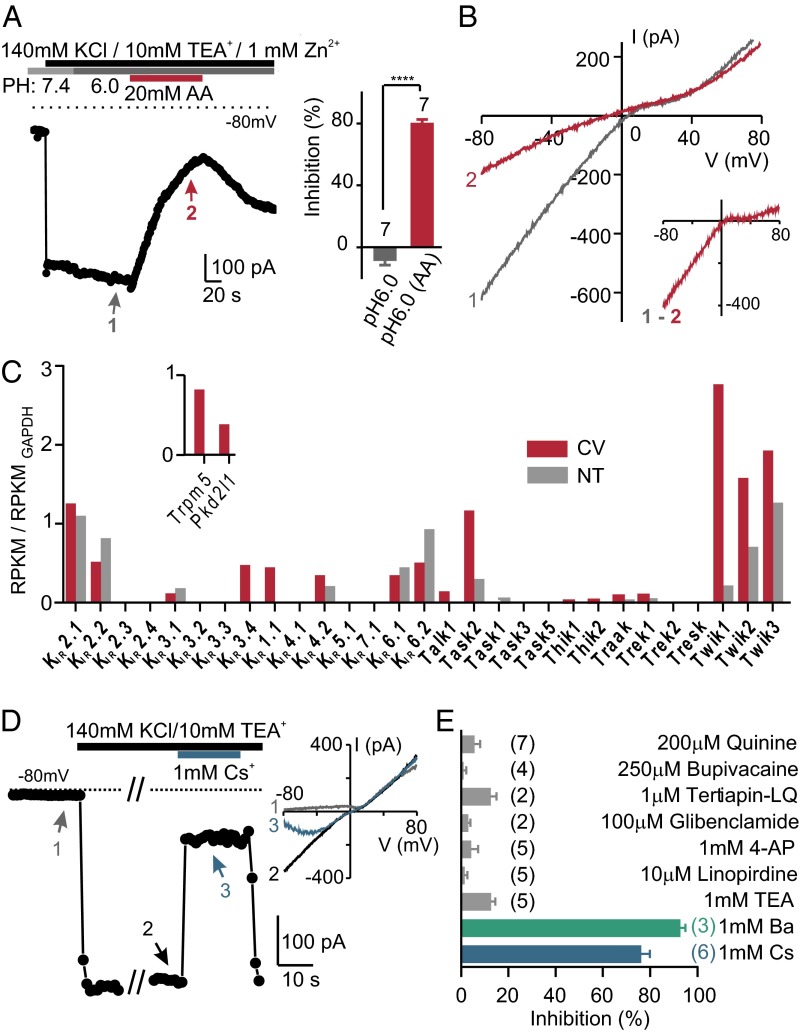
Fig. 2.
An inward rectifier K+ current in PKD2L1 cells is inhibited by intracellular acidification. (A) The inward K+ current measured in a PKD2L1 cell was reversibly inhibited by 20 mM acetic acid, pH 6.0, but not by pH 6.0 alone. Average data are shown in the Inset. ****P < 0.0001 using paired two-tailed Student’s t test. (B) I–V relation measure at the times indicated in A. Note that the difference current shows inward rectification. (C) The normalized expression levels of Kcnj (KIR) and Kcnk (K2P) transcripts from deep sequencing of taste and nontaste tissues (average of two samples each). The mapped reads were used to compute reads per kilobase per million reads (RPKM) for each gene, which was further normalized to the RPKM of GAPDH in each sample. Inset shows expression level of two taste cell markers, Trpm5 and Pkd2l1, which were detected only in taste tissue. (D) The inward K+ current from a PKD2L1 cell was reversibly blocked by 1 mM Cs+. Inset shows I–V relation measured at the time points indicated. (E) K+ channel blockers were tested against resting K+ currents in PKD2L1 cells in 100 mM NaCl, 50 mM KCl. The number of cells tested is indicated next to the bars. Of the compounds tested, only 1 mM Ba2+ and 1 mM Cs+ produced a significant block of the inward K+ current (P < 0.0001 by one-way ANOVA followed by Tukey’s post hoc analysis).