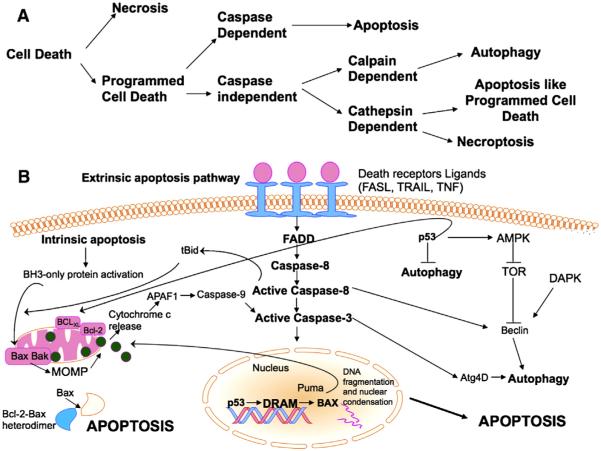
Fig. 1.
The apoptosis pathway: (A) The different paths that a cell can take during the activation of cell death. (B) Apoptosis can be triggered either by external receptor-dependent stimulus (extrinsic) or through internal (intrinsic) mitochondria-mediated signaling. The extrinsic pathway is initiated by the attachment of death receptors with their death initiating ligands, such as FASL, TRAIL or TNF. Consequently, an adaptor molecule, FADD also known as FAS-associated death domain protein, couples the death receptors and this subsequently leads to the activation of caspase-8. Activated caspase-8 can either directly cleave and activate caspase-7 or caspase-3, thereby promoting apoptosis. On the other hand the intrinsic pathway is modulated by the activation of BH3-only proteins sensing different types of cell stress, such as DNA damage or ER stress, and then activating BAX/BAK at mitochondrial outer membrane (MOM). MOM permeabilization (MOMP) leads to release of different apoptosis-mediating molecules, such as cytochrome c, which activates caspase-9. In turn, caspase-9 cleaves and activates caspase-3 and caspase-7, thus triggering apoptotic cell death. Both pathways interface at the point of caspase-3 activation. The formation of autophagosome formation requires activation of Beclin 1 which acts as a component of a multiprotein (PI3K) complex. The crosstalk between autophagy and apoptosis is mediated at least in part by the functional and structural interaction between Beclin 1 and the anti-apoptotic proteins BCL-2 and BCL-XL. Diverse apoptotic stimuli either intrinsic or extrinsic can lead to caspase-mediated cleavage of Beclin 1 rendering it ineffective as an autophagy inducer. The master tumor suppressor p53 has essential roles in both apoptosis and autophagy. At the transcriptional level, p53 upregulates BAX, PUMA and BID or reduces the expression of BCL-2, which antagonizes BAX. In addition to apoptosis, p53 can also induce autophagy through TOR inhibition and also through transcriptional activation of DRAM.