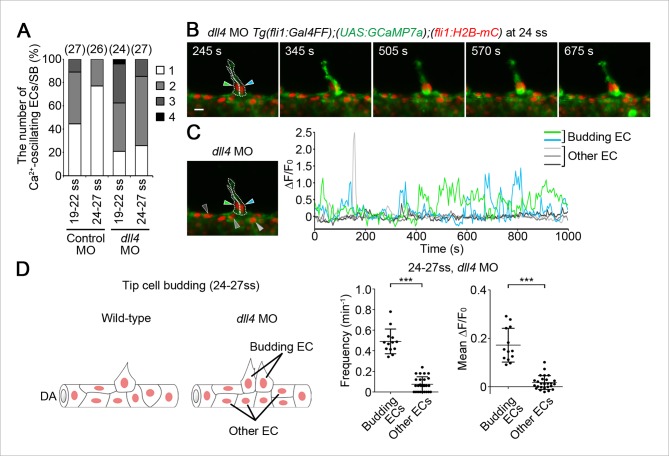
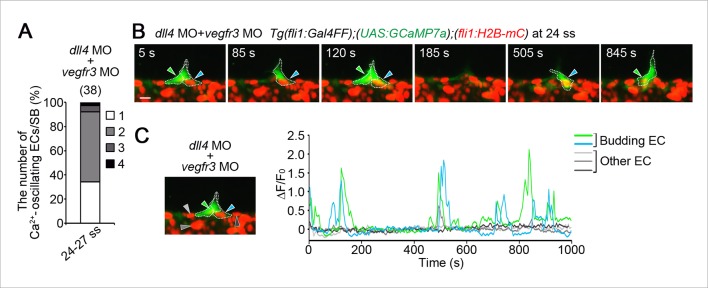
Figure 9. Dll4 is involved in suppressing Ca2+ responses in ECs adjacent to tip cells.
(A) The number of Ca2+-oscillating ECs at each somite boundary of the embryo injected with control MO or dll4 MO was quantified as in Figure 4A. (B) 3D-rendered time-sequential images of Tg(fli1:Gal4FF);(UAS:GCaMP7a);(fli1:H2B-mC) embryos during tip cell budding injected with dll4 MO (24 ss). Green and blue arrowheads indicate two neighboring Ca2+-oscillating ECs, both of which are budding from the DA. (C) The fluorescence changes in GCaMP7a (ΔF/F0) of individual ECs from B indicated by arrowheads at the left panel are shown as a graph. (D) Ca2+ oscillation frequency (left) and mean ΔF/F0 (right) in budding ECs and other ECs within the DA in dll4 morphants during tip cell budding at 24–27 ss as illustrated at the left panel (n ≥ 13). Scale bar, 10 mm in B. ***p < 0.001. DA, dorsal aorta.