Abstract
Objective
This study describes routine HIV screening implementation and outcomes in three hospitals in Chicago, Illinois.
Methods
Retrospective data from three hospitals were examined, and routine testing procedures, testing volume, reactive test results, and linkage-to-care outcomes were documented.
Results
From January 2012 through March 2014, 40,788 HIV tests were administered at the three hospitals: 18,603 (46%) in the emergency department (ED), 7,546 (19%) in the inpatient departments, and 14,639 (36%) in outpatient clinics. The screened patients varied from 1% to 22% of the total eligible patient population across hospitals. A total of 297 patients tested positive for HIV for a seropositivity rate of 0.7%; 129 (43%) were newly diagnosed and 168 (57%) were previously diagnosed, with 64% of those previously diagnosed out of care at the time of screening. The inpatient areas had the highest seropositivity rate (0.6%). The percentage of newly diagnosed patients overall who were linked to care was 77%. Of newly diagnosed patients, 51% had ≥1 missed opportunity for testing (with a mean of 3.8 visits since 2006), and 30% of patients with missed opportunities were late testers (baseline CD4+ counts <200 cells per cubic millimeter).
Conclusion
Routine screening is an essential tool for identifying new infections and patients with known infection who are out of care. Hospitals need to provide HIV screening in inpatient and outpatient settings—not just EDs—to decrease missed opportunities. Routine screening success will be driven by how notification and testing are incorporated into the normal medical flow, the level of leadership buy-in, the ability to conduct quality assurance, and local testing laws.
In 2006, the Centers for Disease Control and Prevention (CDC) recommended routine, opt-out HIV screening in health-care settings for patients aged 13–64 years.1 In 2013, the U.S. Preventive Services Task Force gave an A recommendation for routine testing, making it easier for medical providers to obtain reimbursement for HIV tests.2 Still, many clinicians do not routinely screen patients for HIV, and more than 50% of U.S. adults have never been screened.3 Early diagnosis of HIV infection decreases mortality in patients who initiate treatment and reduces health-care costs;4 people on antiretrovirals reduce their risk of transmission by 96%.5 People unaware of their HIV status will likely have a shorter life span and are responsible for much of the spread of HIV infection;6 routine screening and linkage to care are as important for the person with undiagnosed HIV as for those he or she might infect.
Although significant research has been undertaken on routine HIV screening in emergency departments (EDs),7 more research is needed on hospital and outpatient settings.8 Routine HIV screening has been shown to achieve better patient and public health outcomes when it includes significant changes in institutional policy, normalization of HIV screening standards with other diagnostic care services, modifications to emergency medical records (EMRs) that more effectively prompt testing to occur, and an effective method to track patient health outcomes.9–11 CDC recently supported these pillars of routine testing.10 Besides new infections, routine screening identifies people who are aware of their status but not in care. However, the literature has not thoroughly explored linkage to care for this population.
The justifications for routine screening and the approach to integrating screening into medical care are becoming clear, but challenges remain. The majority of states have not met the following national testing goals: (1) increase by 4% the percentage of people ever tested for HIV, (2) reduce by 25% the percentage of people diagnosed with acquired immunodeficiency syndrome (AIDS) within three months of an HIV diagnosis, and (3) increase to 85% the percentage of people who are linked to HIV medical care within three months of diagnosis. For example, Illinois ranks 35th among 50 states and Washington, D.C., for people tested for HIV and 22nd for people with late-stage diagnosis.12 Medical providers have competing priorities when treating patients, HIV testing laws and interpretations of CDC's recommendations vary greatly, resources for HIV screening are limited, and stigma related to HIV remains pervasive among health-care professionals and the populations they serve.13 In a routine screening model, health-care providers must address patients' immediate needs while also screening for HIV, easing their anxieties about diagnosis, and offering resources to link them to care.
This study examined the implementation of routine HIV screening in three hospitals in Chicago, Illinois; their success in finding new infections and reducing missed opportunities for diagnosis; and how people with known HIV infection were identified and linked to care. In 2013, Chicago had an HIV incidence rate (40 per 100,000 population) that was two-and-a-half times the national rate (15 per 100,000 population) and an HIV prevalence (828 per 100,000 population) that was almost three times the national rate (292 per 100,000 population). More than 3,000 Chicago residents live with undiagnosed HIV.14
METHODS
Setting
We selected three Chicago hospitals that participated in the Frontlines of Communities in the United States (FOCUS) program to be part of this analysis:
Chicago Advocate Trinity Hospital: a private hospital located in Calumet Heights, which in 2012 had an HIV prevalence rate of 579 per 100,000 population and an annual HIV incidence rate of 40 per 100,000 population.14
Sinai Health System: a designated safety-net provider located in North Lawndale, which in 2012 had an HIV prevalence rate of 1,030 per 100,000 population and an HIV incidence rate of 68 per 100,000 population.14
University of Chicago Medicine: a private academic medical center located in Hyde Park, which in 2012 had an HIV prevalence rate of 561 per 100,000 population and an HIV incidence rate of 27 per 100,000 population.14
To maintain a certain level of anonymity for these hospitals, we refer to them as Hospitals A, B, and C.
Patient selection
All patients selected for the analysis were screened for HIV from January 2012 to March 2014 in Hospitals A and C, and from July 2013 to March 2014 in Hospital B (its program started in July 2013). HIV screening occurred in the ED, inpatient floors, and outpatient clinics in Hospitals A and C; Hospital B limited routine HIV screening to the ED. All patients provided oral or written consent, were aged 13–64 years (>16 years of age for Hospital C, as younger patients are seen in Hospital C's children's hospital), were not known to be HIV positive, had blood drawn prior to leaving the institution, were mentally stable, and were not experiencing a life-threatening issue when offered screening.
Overview of routine HIV testing model
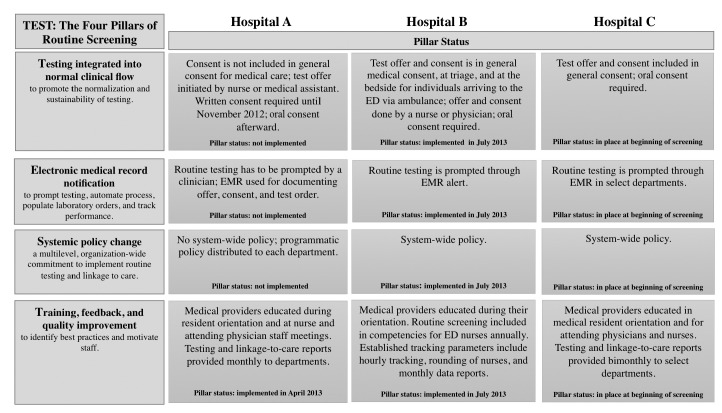
The hospitals are part of the FOCUS program, which has established more than 150 partnerships in 11 U.S. cities, resulting in more than 1 million HIV tests and more than 5,400 HIV diagnoses.10 FOCUS is implemented via four pillars of routine testing implementation: (1) testing integrated into normal clinical flow, (2) EMR modification, (3) system policy change, (4) and training, feedback, and quality improvement (Figure 1).10 All three hospitals included routine HIV screening expectations into staff trainings, shared tracking reports (weekly for Hospitals B and C and monthly for Hospital A), and adapted their EMRs; two hospitals (Hospitals A and B) provided updates on routine screening efforts in clinical staff meetings.
Figure 1.
The four pillarsa of routine HIV screening implemented at three hospitals in Chicago, Illinois, 2012–2014
aSource: Lin X, Dietz PM, Rodriguez V, Lester D, Hernandez P, Moreno-Walton L, et al. Routine HIV screening in two health-care settings— New York and New Orleans, 2011–2013. MMWR Morb Mortal Wkly Rep 2014;63(25):537-41.
HIV = human immunodeficiency virus
ED = emergency department
EMR = electronic medical record
Test offers, consent, and counseling
Illinois State law permits oral consent for an HIV test and requires that a medical provider conduct the HIV test.15 The test offer process varied by hospital. Oral and written consent were used during the analysis period. For Hospital A, written consent was no longer required by the hospital after November 2012. Oral consent was documented at all three hospitals. Patients at Hospitals A and B received negative results and posttest counseling from a medical provider prior to discharge. Hospital C provided posttest counseling for patients who tested positive after discharge.
Testing procedures
All hospitals used either fourth-generation testing, the ARCHITECT® Ag/Ab Combo Assay (Abbott Laboratories, Abbott Park, Illinois) (testing for the p-24 antigen), or enzyme-linked immunosorbent assay (testing for antibodies). For part of the time period, specimens testing positive for antibodies were then tested at an external site utilizing the Western blot test. By 2014, all hospitals used the Multispot HIV-1/HIV-2 Rapid Test (Bio-Rad Laboratories, Hercules, California) as a supplemental test on specimens with the p-24 antigen or HIV antibody. When acute infection was suspected (Multispot negative or indeterminate), a viral load test was administered.
Missed opportunities
We defined a missed opportunity for a previous HIV diagnosis as a newly diagnosed person having a previous visit at the hospital where HIV diagnosis occurred since October 2006 (after the release of CDC's recommendations)1 with no HIV test conducted.
Linkage to care
All hospitals offered in-person or telephone-based result notification and linkage to care to patients by a dedicated staff member. Hospital C used telephone notification to ensure that patients who were discharged before the test result was available were notified of their results. Linkage to care included result notification and posttest counseling, needs assessment, an HIV primary care appointment, reminder calls, and meeting the patient at the appointment. An individual was considered linked to care if the person attended an HIV primary care appointment within 90 days of receiving his or her result. Patients linked to any HIV primary care provider after 90 days were classified as not linked to care.16
Data collection and analysis
Data collection included the test date, patient name, department, test result, and—for those with new reactive results—prior visit date, previous HIV test result, CD4 count, viral load, medical appointment date, and medical appointment attainment. Testing data for the 12 months prior to the beginning of data collection were also provided for each hospital.
We used Pearson's chi-squared test for associations between categorical variables. We also used one-way analysis of variance to examine associations between categorical and continuous variables. Individual logistic regression models examined the relationships among status at the time of testing, patient demographics, and testing department. Because of the association of risk and linkage-to-care outcome, we included risk as a covariate in the logistic regression model examining the effect of the testing site on linkage to care. We considered p<0.05 to be statistically significant. All data were analyzed using Stata® release 12.17
RESULTS
The three hospitals collectively performed 40,788 HIV tests. The percentage of patients eligible for screening varied among sites from 1% to 22% of unique patients screened for HIV. Of patients who were eligible and tested, 297 (0.7%) were seropositive and 129 (43%) were determined to be new diagnoses. The remaining 168 (57%) were previously diagnosed with HIV (Table 1). Of 168 patients who were previously diagnosed, 61 (36%) reported being in HIV care at the time of screening or shortly thereafter (Table 2).
Table 1.
Testing information and seroprevalence among all patients routinely screened for HIV, by hospital and medical department, three hospitals, Chicago, Illinois, 2011–2014
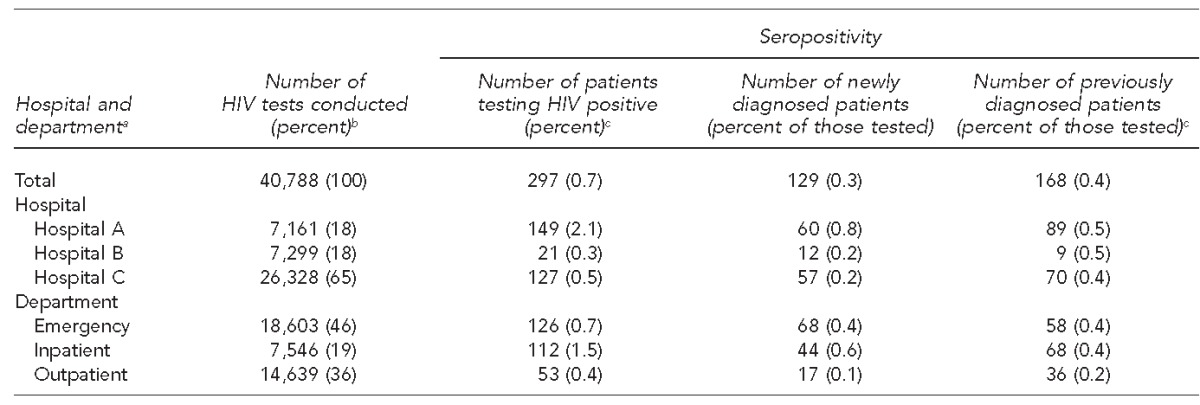
aTesting information for each department across all hospitals
bPercentages may not sum to 100 because of rounding.
cTesting department was not reported for six previously diagnosed patients.
HIV = human immunodeficiency virus
Table 2.
Linkage-to-care outcomes for people who were newly and previously diagnosed with HIV during implementation of routine HIV screening, three hospitals, Chicago, Illinois, January 2012–March 2014
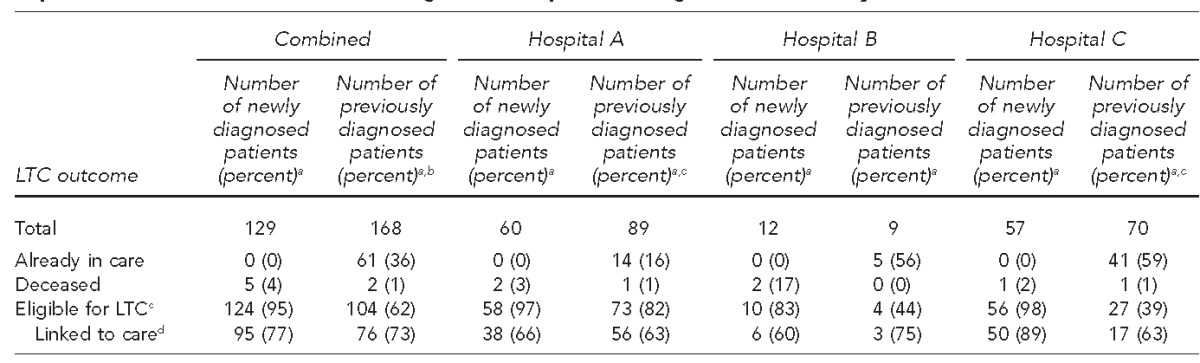
aPercentages may not total to 100 because of rounding.
bLinkage-to-care data were incomplete for one previously diagnosed patient.
cPatients were eligible for linkage to care if they were living in the Chicagoland area at the time of linkage, alive, and not incarcerated when linkage attempts were made. One patient in Hospital A and one patient in Hospital C were not eligible for linkage to care.
dThe denominator for percent linked to care is the number of patients eligible for linkage to care.
HIV = human immunodeficiency virus
LTC = linkage to care
Testing volume and department prevalence
The number of tests by site during the study period ranged from 480 to 3,363 tests per quarter, representing the scale-up that occurred toward routine screening (Figure 2). Hospital A showed the greatest increase (234%), from 480 tests in the first quarter of 2012 to 1,605 tests in the first quarter of 2014. Hospitals B and C also saw increases in the number of patients tested, although the increases were much lower than that of Hospital A. The number of tests at Hospital C increased from 1,495 to 2,336 (56%) from the first quarter of 2012 to the first quarter of 2014. The number of tests at Hospital B increased from 2,724 to 2,907 (7%) from the first quarter of 2013 to the first quarter of 2014. Hospitals B and C had all four pillars implemented at the start of routine screening (Figure 1). Hospital A is still implementing pillars two and three.
Figure 2.
Total routine HIV tests and important milestones at three hospitals in Chicago, Illinois, 2011–2014
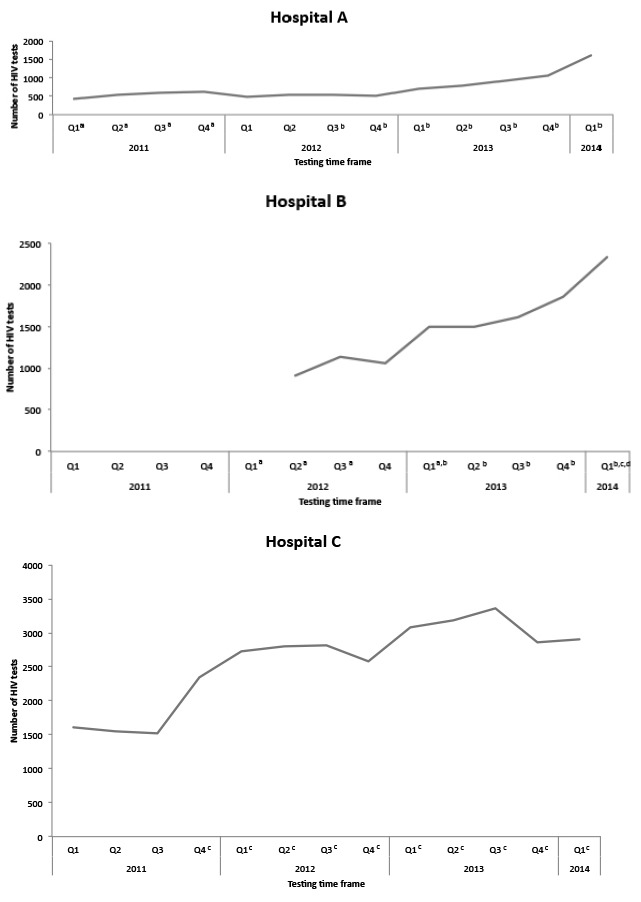
aHealth educator testing occurred. HIV screening was performed by both clinicians and health educators, who were certified HIV test counselors and responsible for testing patients in the hospital.
bNurse-driven, triage-integrated model. At triage, nursing staff members consented patients for HIV screening.
cOral consent permitted. Patients were able to provide oral consent to clinical care staff, without written consent.
dHourly rounding initiated. Project coordinators monitored HIV screening through the use of electronic medical records on an hourly basis to improve screening rates for all eligible patients.
HIV = human immunodeficiency virus
In the data collected, seropositivity and the number of tests varied by department (Table 1). Of the 40,788 tests reported in this analysis, EDs conducted 18,603 (46%), outpatient clinics (e.g., primary care and subspecialty clinics) conducted 14,639 (36%), and inpatient care sites conducted 7,546 (19%). However, the positivity rate for tests conducted at inpatient care sites (1.5% overall, 0.6% for new positives) was higher than that of EDs (0.7% overall, 0.4% for new positives) and outpatient clinics (0.4% overall, 0.1% for new positives).
Newly diagnosed patients
Overall, 129 patients were newly diagnosed with HIV infection through these initiatives (Table 1), and eight of them (6%) had acute HIV infection. Patients newly diagnosed as HIV positive through screening differed from patients who were previously diagnosed with HIV only by being younger (13–29 years of age) at the time of testing (p<0.001) (Table 3).
Table 3.
Selected characteristics of patients testing HIV positive during implementation of routine HIV screening at three hospitals in Chicago, Illinois, January 2012 through March 2014
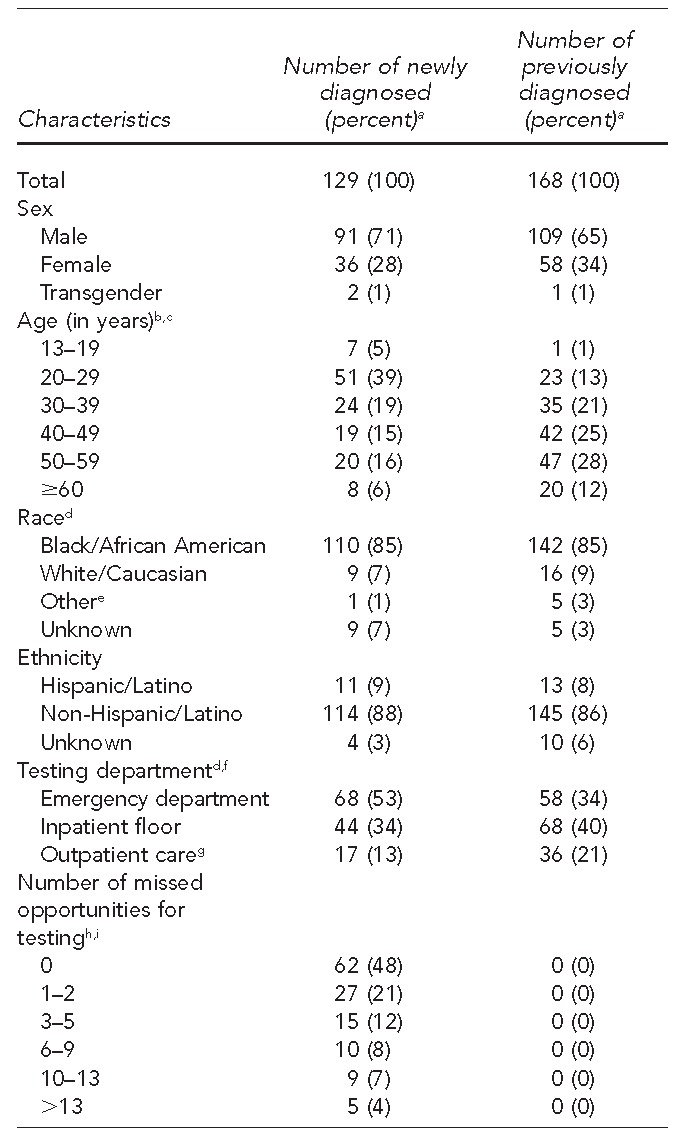
aPercentages may not total to 100 because of rounding.
bUnadjusted p<0.001 using Pearson's chi-squared test
cAdjusted p<0.001 (model includes age, race, and testing department) using logistic regression
dUnadjusted p<0.05 using Pearson's chi-squared test
e“Other” includes Asian, Native Hawaiian/other Pacific Islander, and multiracial.
fSix patients did not have testing department information.
gOutpatient care includes primary, obstetrician/gynecologist, and specialty care clinics.
hMissed opportunities are only for patients who are newly diagnosed.
iOne patient was missing data on missed opportunities.
HIV = human immunodeficiency virus
The overall linkage-to-care rate was 77%. Five patients who died during the 90-day time period were removed from related analyses. Patients who self-identified as heterosexual were less likely to be linked to care than other risk groups (p=0.039). Other demographic variables did not differ for those who were and were not linked to care. The initial relationship between the site of diagnosis and linkage to care—found to be significant—was no longer significant after including risk factor in the model.
Baseline CD4+ counts ranged from 2 cells per cubic millimeter (cells/mm3) to 1,238 cells/mm3. Of 111 patients with documented CD4+ counts, 38 (34%)were late testers (baseline CD4+ count ≤200 cells/mm3).
Missed opportunities
Among 289 hospital visits for newly diagnosed patients, 195 (64%) were from the ED, 48 (16%) were from inpatient units, and 46 (15%) were from specialty care. Of the newly diagnosed patients, 51% had ≥1 missed opportunity for testing, with a mean of 3.8 visits since 2006 and a mean baseline CD4+ count of 335 cells/mm3. Thirty percent of patients with missed -opportunities were late testers (baseline CD4+ counts <200 cells/mm3); however, we found no significant difference between those who were and were not late testers.
Previously diagnosed patients
Across the hospitals, of 168 patients who were screened, 104 (62%) were previously diagnosed but not eligible for linkage to care because they were already in care, 61 (36%) reported being in care at the time of testing, and two (1%) were deceased (Table 2). Forty percent of previously diagnosed patients were screened in inpatient units. Women were significantly more likely than men to be out of care at the time of retesting (p=0.034). Patients who reported that they were in care at the time of testing did not differ in any other way from those who were out of care. Of the 104 patients who required linkage to care, 76 (73%) received it (Table 2).
DISCUSSION
The existence of all four pillars when Hospitals B and C began routine testing facilitated screening a greater number of patients. Hospitals B and C had a system-wide routine testing policy and consent for HIV testing incorporated into the general consent for care when routine screening was initiated. Hospital B integrated hourly rounding at the initiation of routine screening, using an existing quality assurance process to keep patients informed of screening availability. Hospital B also ascertained through the EMR whether or not eligible patients had been offered a test and had a physician's standing order allowing the HIV test to be consented and ordered by a registered nurse, advanced practice nurse, or physician assistant; as such, missed opportunities could be addressed in real time.
Hospital A's routine screening relied on a clinician to offer an HIV test, a programmatic vs. system-wide policy, limited executive buy-in, and an EMR that did not allow clinicians to easily consent patients as part of the normal clinical flow. Hospital C had limited capacity to provide departmental feedback and EMR alerts that did not transmit universally. These challenges resulted in Hospitals A and C screening ≤5% of unique patients each quarter.
Departmental prevalence
Inpatient units offer an opportunity to identify patients who, because of their level of acuity or because their presenting concern takes precedence, were not tested in the ED. Inpatients are often there for several days and are seen by a number of care providers, thereby increasing their chances of being tested. When they are diagnosed or reidentified, opportunities exist to improve linkage to care after discharge; however, such linkages can be difficult, as patients often feel better, have multiple follow-up appointments, and might not have regular involvement in primary or preventive care.
Those in outpatient settings might access care routinely, creating an opportunity for HIV testing. As most programs have focused on ED testing, individuals receiving care in outpatient settings are less likely to receive HIV testing unless patients specifically request a test. By expanding testing to all medical settings, the location where patients access care will no longer be a barrier to receiving testing. The seropositivity rate for outpatient clinics was only 0.1% for newly diagnosed patients, but this seroprevalence rate meets CDC's guidelines for the recommended prevalence in health-care settings of ≥0.1%. Given the number of patients who can be screened as outpatients (14,639 test events), we also might reduce the stigma attached to HIV testing.
Linkage to care of previously diagnosed patients
Routine HIV screening gives previously diagnosed patients not actively involved in HIV primary care the opportunity to discuss their status with a physician and be linked to care. In Chicago, 7,458 of 20,067 (37%) people diagnosed with HIV are not retained in care;14 in our analysis, of the 297 screened patients who were aware of their status, 104 (35%) were not in care. These patients typically present in the ED and are often admitted into an inpatient unit because they are not in care and, consequently, get sick.18 Routine inpatient screening gives physicians time to discuss patients' disease state with them and emphasize the importance of care. In our analysis, the overall linkage-to-care rate was 75%, which was high given the difficulty of linking these individuals to care. Research is available on how to engage patients through routine screening programs and the challenges associated with doing so, but there is a need for greater focus on this population.18,19
Linkage to care of newly diagnosed patients
Linkage to care, a cornerstone of any effective routine screening program, is a challenge in Chicago and nationwide. Agencies implementing routine testing might vary in whether or not a clinician or dedicated staff member will provide linkage to care, but we have seen that it is more effective if it is the responsibility of someone within the institution. In our analysis, 95 of 129 (77%) newly diagnosed patients were linked to care within 90 days of diagnosis. Rates varied by hospital (66% at Hospital A, 60% at Hospital B, and 89% at Hospital C). The higher percentage of patients linked to care at Hospital C might have been due to the telephone notification procedure of Hospital C, where the social worker responsible for notification has extensive experience in the HIV field and has developed skills that allow him or her to connect well with patients by telephone. Telephone-based notification is an area in need of future exploration, as it might be a viable option for outpatient and ED routine screening programs where patients might be unwilling or unable to return for their result. It is not ideal in cases of acute infection, as both the patient and the community benefit from immediate, on-site linkage to care. Additionally, Hospital A had many patients who were hospitalized for extended periods of time, making it difficult to link them to care within 90 days.
Legal considerations
It is important to consider the legal environment in Illinois to understand the success or challenges of implementing routine HIV testing at these hospitals. True opt-out testing would permit consent for HIV testing simply to be assumed as part of the general consent for medical care process where notification of the test is given.1 However, the Illinois AIDS Confidentiality Act (updated in 2008 after the release of CDC's recommendations) requires documented consent, which can be conservatively interpreted as separate written or verbal consent.10 In Hospital A, written consent was required to test someone for HIV until November 2012; after verbal consent was permitted, the hospital saw a >100% increase in testing system-wide. States such as Louisiana20 have a more literal translation of CDC's recommendations and have seen great success in their routine testing programs. Modifying state laws to better reflect CDC recommendations might help increase testing volumes in clinical settings. Managers from each hospital study worked collaboratively with state legislators to amend the Illinois AIDS Confidentiality Act to facilitate opt-out screening after the completion of this study.21
Limitations
This study was subject to several limitations. For one, these programs were developed as part of patient care, not with research in mind. As such, the availability of patient risk factors for HIV, CD4 counts upon diagnosis, and other variables depended on the care provider documenting the information in the EMR and information obtained by the navigator. The limited -pre-program data and the number of truly eligible patients made it difficult to gauge the success of programs that had been testing prior to 2012. Also, using 2006 as our cutoff for missed opportunities made it more difficult to compare our results with the results of other studies. Finally, our analysis might not be generalizable to other medical settings. Hospital culture and structure vary widely and can either facilitate or hinder different components of a screening program.
CONCLUSIONS
Without routine HIV testing in clinical settings, we cannot achieve the National HIV/AIDS Strategy objectives related to HIV testing and linkage to care.16 The three hospitals in this study had varying success in implementing routine HIV testing due to institutional characteristics and implementation of the four FOCUS pillars. The pillars seem to have the biggest impact if in place at the same time prior to starting the testing program. Additionally, it is important to consider how notification and testing are incorporated into the normal medical flow, the type of institution, the level of leadership buy-in, the ability to conduct quality assurance, and thorough knowledge of local testing laws. The hallmarks of an effective routine HIV testing program are a high proportion of eligible patients being screened, a low late-stage diagnosis rate (<19%), and at least 85% of patients being linked to care. Consent for HIV testing as part of the general consent and screening integrated at triage, the bedside, and discharge will exhaust opportunities for identifying infections. Agencies should collaborate to benefit from others' lessons to optimize the effectiveness of their routine testing efforts.
Footnotes
The projects described were partially supported by funding from Gilead Sciences, Inc. The authors acknowledge the feedback and support of Lora Branch, Gilead Sciences, Inc.; Clara Bertozzi-Villa, University of Chicago Medicine; John Schneider, MD, University of Chicago Medicine; Michelle Gaskill, RN, MHA, Advocate Trinity Hospital; Claude Hall, Sinai Health System; Erik Schwab; and the graphic design assistance of McBee Strategic Consulting.
This study was reviewed by the institutional review board for each hospital and all were given a waiver to use the data in this analysis.
REFERENCES
- 1.Branson BM, Handsfield HH, Lampe MA, Janssen RS, Taylor AW, Lyss SB, et al. Revised recommendations for HIV testing of adults, adolescents, and pregnant women in health-care settings. MMWR Recomm Rep. 2006;55(RR-14):1–17. [PubMed] [Google Scholar]
- 2.Moyer VA U.S. Preventive Services Task Force. Screening for HIV: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2013;159:51–60. doi: 10.7326/0003-4819-159-1-201307020-00645. [DOI] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (US) Atlanta: Department of Health and Human Services (US), CDC; 2013. HIV testing trends in the United States, 2000–2011. Also available from: http://www.cdc.gov/hiv/pdf/testing_trends.pdf [cited 2015 Jun 9] [Google Scholar]
- 4.Johnson S, Heitgerd J, Koenig LJ, VanHandel M, Branson BM, Connelly E, et al. Vital signs: HIV testing and diagnosis among adults—United States, 2001–2009. MMWR Morb Mortal Wkly Rep. 2010;59(47):1550–5. [PubMed] [Google Scholar]
- 5.Donnell D, Baeten JM, Kiarie J, Thomas KK, Stevens W, Cohen CR, et al. Heterosexual HIV-1 transmission after initiation of antiretroviral therapy: a prospective cohort analysis. Lancet. 2010;375:2092–8. doi: 10.1016/S0140-6736(10)60705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Marks G, Crepaz N, Janssen RS. Estimating sexual transmission of HIV from persons aware and unaware that they are infected with the virus in the USA. AIDS. 2006;10:1447–50. doi: 10.1097/01.aids.0000233579.79714.8d. [DOI] [PubMed] [Google Scholar]
- 7.Knapp H, Hagedorn H, Anaya HD. HIV rapid testing in a Veterans Affairs hospital ED setting: a 5-year sustainability evaluation. Am J Emerg Med. 2014;32:878–83. doi: 10.1016/j.ajem.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 8.Maxwell CJ, Sitapati AM, Abdus-Salaam SS, Scott V, Martin M, Holt-Brockenbrough ME, et al. A model for routine hospital-wide HIV screening: lessons learned and public health implications. J Natl Med Assoc. 2010;102:1165–72. doi: 10.1016/s0027-9684(15)30771-9. [DOI] [PubMed] [Google Scholar]
- 9.Goetz MB, Hoang T, Knapp H, Burgess J, Fletcher MD, Gifford AL, et al. Central implementation strategies outperform local ones in improving HIV testing in Veterans Healthcare Administration facilities. J Gen Intern Med. 2013;28:1311–7. doi: 10.1007/s11606-013-2420-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sanchez TH, Sullivan PS, Rothman RE, Brown EH, Fitzpatrick LK, Wood AF, et al. A novel approach to realizing routine HIV screening and enhancing linkage to care in the United States: protocol of the FOCUS program and early results. JMIR Res Protoc. 2014;3:39. doi: 10.2196/resprot.3378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Dietz PM, Rodriguez V, Lester D, Hernandez P, Moreno-Walton L, et al. Routine HIV screening in two health-care settings—New York City and New Orleans, 2011–2013. MMWR Morb Mortal Wkly Rep. 2014;63(25):537–41. [PMC free article] [PubMed] [Google Scholar]
- 12.Dietz PM, Krueger AL, Wolitski RJ, Johnson AS, Dunbar E, Lin SL, et al. Atlanta: Centers for Disease Control and Prevention (US); 2014. State HIV prevention progress report, 2014. Also available from: http://www.cdc.gov/hiv/pdf/policies/StateProgressReport2014.pdf [cited 2015 Jun 9] [Google Scholar]
- 13.Bartlett JG, Branson BM, Fenton K, Hauschild BC, Miller V, Mayer KH. Opt-out testing for human immunodeficiency virus in the United States: progress and challenges. JAMA. 2008;300:945–51. doi: 10.1001/jama.300.8.945. [DOI] [PubMed] [Google Scholar]
- 14.Chicago Department of Public Health. Chicago: City of Chicago; 2013. HIV/STI surveillance report, Chicago, 2013. Also available from: https://www.cityofchicago.org/content/dam/city/depts/cdph/infectious_disease/STI_HIV_AIDS/HIV_STISurveillanceReport2013.pdf [cited 2015 Jun 9] [Google Scholar]
- 15. 410 ILCS 305 (2008)
- 16.The White House (US), Office of National AIDS Policy. National HIV/AIDS strategy for the United States. 2010 [cited 2015 Jun 9] Available from: http://aids.gov/federal-resources/national-hiv-aids-strategy/nhas.pdf.
- 17.StataCorp. Stata®: Release 12 for Windows. College Station (TX): StataCorp; 2013. [Google Scholar]
- 18.Horstmann E, Brown J, Islam F, Buck J, Agins BD. Retaining HIV-infected patients in care: where are we? Where do we go from here? Clin Infect Dis. 2010;50:752–61. doi: 10.1086/649933. [DOI] [PubMed] [Google Scholar]
- 19.Wohl AR, Galvan FH, Myers HF, Garland W, George S, Witt M, et al. Do social support, stress, disclosure, and stigma influence retention in HIV care for Latino and African American men who have sex with men and women? AIDS Behav. 2011;15:1098–110. doi: 10.1007/s10461-010-9833-6. [DOI] [PubMed] [Google Scholar]
- 20. LA Rev Stat § 40:1300.13 (2011)
- 21. 410 ILCS 305 (2015)