Abstract
Little is known regarding the underlying relationship between smoking initiation and current quantity smoked during adolescence into young adulthood. It is possible that the influences of genetic and environmental factors on this relationship vary across sex and age. To investigate this further, the current study applied a common causal contingency model to data from a Virginia-based twin study to determine: (1) if the same genetic and environmental factors are contributing to smoking initiation and current quantity smoked; (2) whether the magnitude of genetic and environmental factor contributions are the same across adolescence and young adulthood; and (3) if qualitative and quantitative differences in the sources of variance between males and females exist. Study results found no qualitative or quantitative sex differences in the relationship between smoking initiation and current quantity smoked, though relative contributions of genetic and environmental factors changed across adolescence and young adulthood. More specifically, smoking initiation and current quantity smoked remain separate constructs until young adulthood, when liabilities are correlated. Smoking initiation is explained by genetic, shared, and unique environmental factors in early adolescence and by genetic and unique environmental factors in young adulthood; while current quantity smoked is explained by shared environmental and unique environmental factors until young adulthood, when genetic and unique environmental factors play a larger role.
Keywords: smoking, genetic and environmental influences, adolescence and young adulthood, VTSABD, common causal contingency model
Cigarette smoking is the leading cause of preventable death in the United States and has been associated with considerable economic, social, and personal costs. Annually, tobacco use costs the nation an estimated $193 billion, inclusive of lost productivity and direct health care expenditures (Centers for Disease Control and Prevention & Office on Smoking and Health, 2008). Yet, 19% of all US adults, or approximately 43.8 million people, smoke cigarettes (Centers for Disease Control and Prevention, 2012). Of these adult smokers, 70% began smoking regularly by the age of 18 (US Department of Health and Human Services, 1994).
Despite notable declines in cigarette smoking over the past 40 years, smoking behavior among adolescents remains a huge public health concern. Every day, about 3,900 children under the age of 18 try their first cigarette. Of these children, an estimated 950 will become new, regular daily smokers (Substance Abuse and Mental Health Services Administration, 2008); approximately half will die as a result of nicotine addiction and other smoking-related causes (Centers for Disease Control and Prevention & Office on Smoking and Health, 2008). Twin studies have suggested that both genetic and environmental factors contribute to smoking behavior. However, many of these twin studies investigated the influences of genes and the environment on cigarette use among adults, so less information is known regarding the genetic and environmental influences of cigarette use in adolescents.
Early twin studies investigating the genetic and environmental influences of smoking behavior of adolescents analyzed various stages of smoking behavior such as initiation, progression, dependence, and addiction separately (Koopmans et al., 1997; 1999; Lyons et al., 2008; Madden et al., 1999). These studies found that the initiation of tobacco use in adolescence was primarily explained by shared environmental factors (Koopmans et al., 1997; Slomowski et al., 2005), while genetic factors contributed more to individual differences in other smoking behaviors, such as daily quantity of cigarettes smoked (Koopmans et al., 1997; 1999) or smoking progression, which has an estimated heritability of 0.80 (Koopmans et al., 1997; Maes et al., 1999; Vink et al., 2005). Furthermore, population-based twin studies provide evidence that genetic influences come to play a larger role in smoking behavior by late adolescence, when the etiological structure of smoking initiation closely resembles that of adult samples (Karp et al., 2005; Kendler et al., 2008).
Among adult samples, heritability estimates for smoking initiation range from 0.32 to 0.78, making it a moderately heritable trait (Broms et al., 2006; Edwards et al., 1995; Heath et al., 1993; 1999; Kendler et al., 1999; Madden et al., 1999; True et al., 1997). On average, estimates are higher in women relative to men (Heath et al. 2002; Li et al., 2003; Madden et al., 1999; Zavos et al. 2012), suggesting that the heritability of smoking initiation may differ by gender. However, this finding has not been replicated across all studies (Kendler et al., 1999).
As a consequence of analyzing smoking behavioral factors separately, we lack information on whether any overlap exists across stages (Broms et al., 2006). Although we know from adult studies that utilize bivariate and trivariate analyses that significant genetic and environmental covariance exists between initiation and dependence (Gillespie et al., 2009; Kendler et al., 1999; Madden et al., 1999; Maes et al., 2004), it remains unclear whether the genetic and environmental factors influencing the relationship between smoking initiation and progression in adulthood are the same across adolescence into early adulthood (Fowler et al., 2007; Koopmans et al., 1999; Zavos et al., 2012). We also do not know if qualitative and quantitative sex differences found in adult samples exist in adolescent samples (Kendler et al., 2005; Zavos et al., 2012).
Thus, this study seeks to answer these questions by examining the relationship between smoking initiation and current quantity smoked from adolescence to early adulthood, determining if qualitative and quantitative sex differences exist in this relationship, and estimating the contributions of genetic and environmental factors to smoking initiation and current quantity smoked in this younger age group.
Materials and Methods
Sample
Data were obtained from the Virginia Twin Study of Adolescent Behavioral Development (VTSABD) and its young adult follow-up, transitions to substance abuse (TSA). The VTSABD is a multi-wave, cohort-sequential prospective study of adolescent psychopathology and its risk factors, in over 1,400 Caucasian juvenile twin pairs aged 8–17 years and their parents (Meyer et al., 1996); greater detail about the ascertained sample have been provided elsewhere (Hewitt et al., 1997). To be included the present study, individual twins had to have responded to questions regarding smoking initiation and current quantity smoked. The total sample size of this study was 2,804 twins (including 632 MZ male twins, 829 MZ female twins, 367 DZ male twins, 389 DZ female twins, and 587 DZ opposite sex twins). Data obtained for the 22–32-year age group (N = 1,074) was obtained from one wave of the TSA, to which all participants of earlier waves of the VTSABD were invited.
Measures
Data from each of the five waves of the VTSABD were merged and then re-categorized into age groups to ensure that there was an adequate sample size (i.e., 12–13 years, 14–15 years, and 16–17 years). However, since there was only one assessment during the age period from 22–32 years, subdividing the TSA sample by age was not warranted. Two main variables of interest were re-coded across each of these age groups: one measuring whether twins had ever smoked at least one whole cigarette and another measuring the current quantity of cigarettes smoked daily. The ‘ever smoke’ variable was binary, coded as 0 for those who had never smoked at least one whole cigarette and 1 for those who had indicated that they had ever smoked at least one whole cigarette. If respondents indicated that they had ‘ever smoked’ in a given age group (i.e., 14–15 years), they would be given a value of 1 for ‘ever smoke’ in that age group and every subsequent age group (i.e., 14–15 years, 16–17 years, and 22–32 years). Otherwise, if the respondents indicated that they had not ‘ever smoked’ across all age groups, they were given a value of 0 for ‘ever smoke’. To measure current quantity smoked, respondents had to indicate the number of cigarettes smoked daily in the past three months. Free responses were coded into three categories. These categories indicated: zero cigarettes smoked daily (‘non-current smoker’), one–five cigarettes smoked daily (‘current, light smoker’), and five or more cigarettes smoked daily (‘current, heavy smoker’). Only responses where twins indicated that they had smoked before under the ‘ever smoke’ variable were included in the quantity of cigarette use variable. Otherwise, responses for individuals who had indicated that they had never tried cigarettes were coded as missing for the quantity of cigarette use variable.
Descriptive Statistics
Prevalence estimates for smoking initiation and quantity are reported using percentages.
Genetic Analyses
All data analyses were conducted using the open-source structural equation modeling software OpenMx (Boker et al., 2011; Neale et al., 2003). Due to the inadequate sample size for smoking quantity in 12–13-year olds, only univariate genetic analysis on smoking initiation was conducted in this age group. Causal-common-contingent (CCC) models were fit, individually, for smoking initiation and smoking quantity across all other age groups (i.e., 14–15 years and 16–17 years in the VTSABD, and 22–32 years in the TSA).
Using the CCC model originally developed by Kendler and colleagues (1999), smoking behavior was conceptualized as a two-stage process incorporating initiation and current quantity smoked. This model was chosen because it allows for estimating the relative magnitude of the contributions of genetic and environmental factors to smoking liability, as well as for testing the strength of the association between initiation and current quantity smoked stages for smoking via a beta pathway between the two stages (Agrawal et al., 2005; Fowler et al., 2007; Kendler et al., 1999; Maes et al., 2004; Neale et al., 2006).
The significance of an estimated beta pathway between the two stages is used to assess whether the two stages are independent or correlated processes. Specifically, if an estimated beta coefficient is found to be not significant, the liabilities for initiation and current quantity smoked are said to be independent of one another, implying that smoking initiation and current quantity smoked have separate genetic and environmental risk factors. Otherwise, if the estimated beta coefficient is significant, the liabilities for smoking initiation and current quantity smoked are said to share genetic and environmental risk factors. In this case, the beta coefficient provides an estimate of the magnitude of strength of association between smoking initiation and current quantity smoked. The greater the estimated beta coefficient, the larger the magnitude of the strength of the association between smoking initiation and current quantity smoked (i.e., a beta coefficient of zero suggests that the two stages do not share genetic and environmental risk factors, while a beta coefficient of one suggests that the genetic and environmental risk factors for these two stages are identical). The estimated 95% confidence intervals around the beta coefficient give further information regarding the degree of overlap between the two stages. Again, lower limits approaching zero (or below) support independent liabilities and upper limits approaching one provide support for identical liabilities.
Using this model also allows for the direct estimation of additive genetic effects (a2), shared/common environmental effects (c2), and unique environmental effects (e2) on both smoking initiation and current quantity smoked. However, since current quantity smoked is modeled conditionally upon smoking initiation, the genetic and environmental influences unique to current quantity smoked are estimated after those on initiation are taken into account. Thus, the proportion of variance in current quantity smoked explained by the respective influences on initiation can be calculated by multiplying them by the squared beta coefficient. The proportion of the variance in liability to current quantity smoked that is explained by genetic factors is the sum of the proportion of variance in initiation explained by genetic factors multiplied by the squared beta parameter and the proportion of variance explained by unique genetic factors contributing only to the current quantity smoked stage, with the same principle applied for environmental factors.
Nested models were fitted to test specific hypotheses about the nature of association between the two stages of smoking initiation and current quantity smoked. More explicitly, to determine whether qualitative sex differences exist in the relationship between smoking initiation and current quantity smoked, we tested the significance of the genetic and shared environmental correlations between male and female factors. A model constraining the correlation between males and females to one, suggesting that the same factors contribute to male and female smoking behavior, was compared to a model that freely estimated correlations between male and female factors, suggesting that different factors contribute to male and female smoking behavior. This was done separately to test whether the same genes or same environmental factors contribute to the liability of smoking initiation and current quantity smoked in males and females.
Quantitative sex differences were tested for simultaneously to answer the question of whether genetic and environmental factors explain the same proportion of the liability of smoking initiation and current quantity smoked in males and females. To test for quantitative sex differences, a model equating all parameters (i.e., genetic, shared environmental, and unique environmental factors, but not thresholds) for males and females was compared to one allowing for free estimation of parameters for males and females separately. If the model equating parameters between males and females fit the data best, it was concluded that quantitative sex differences did not exist. This process was repeated for each age group.
Following these tests for qualitative and quantitative sex differences, other alternative models were fitted to the data. Specifically, nested models were created to test if there is a direct relationship between smoking initiation and current quantity smoked and whether genetic or common environmental factors could be dropped from initiation and current quantity smoked stages. Where the beta pathway could be dropped from the model without significant loss to goodness-of-fit to the data, it was determined that smoking initiation and current quantity smoked had independent liabilities. Alternatively, when dropping the beta pathway led to significant loss to goodness-of-fit, smoking initiation and current quantity smoked were said to have shared liabilities. Regardless of whether this finding was significant, we moved on to test whether we could drop genetic or shared environmental factors from either initiation or current quantity smoked. Where genetic or environmental factors could not be dropped without significant loss to goodness-of-fit, the factor was said to contribute significantly to the smoking phenotype.
Nested models were compared using likelihood ratio chi-square (LRC) statistics, in which the degrees of freedom equal the difference between the degrees of freedom of the full and nested submodels. LRC is calculated as the difference in −2 log likelihood (−2LL of a comparison model and the −2LL of a reduced nested model) (Neyman & Pearson, 1928; Vuong, 1989). Where the LRC comparing the two models is non-significant, the reduced model is selected as the better fitting model. Akaike information criterion (AIC) was also used as an index of model fit, as well as an index of parsimony (Akaike, 1987; Williams, 1994).
Results
Smoking Prevalence
At age 12–13 years, 10.4% of the total sample had indicated that they had ever smoked. This increased to 27.4% by age 14–15 years, 46.6% by age 16–17 years, and 79.1% by age 22–32 years. Across all age groups, most respondents indicated that they were not current smokers (i.e., indicated that in the past 3 months they smoked zero cigarettes daily). Although the majority (approximately 71%) of adolescents who tried smoking did not become ‘current, heavy smokers’, the proportion of ‘current, light smokers’ and ‘current, heavy smokers’ did increase consistently from the younger to the older age groups (see Table 1).
TABLE 1.
Smoking Initiation and Progression Prevalence of Sample
| % Initiated smoking (indicated having ever smoked)
|
||||
|---|---|---|---|---|
| Age 12–13 | Age 14–15 | Age 16–17 | Age 22–32 | |
| Total sample | 10.4 | 27.4 | 46.6 | 79.1 |
| MZ males | 12.0 | 32.0 | 48.0 | 77.5 |
| MZ females | 8.6 | 22.2 | 35.0 | 76.6 |
| DZ males | 11.2 | 31.3 | 46.8 | 84.2 |
| DZ females | 8.0 | 26.8 | 32.6 | 78.5 |
| DZ opposite sex | 12.4 | 27.8 | 50.2 | 82.6 |
| % Non-current smokers (0 cigarettes smoked daily) | ||||
| Age 12–13 | Age 14–15 | Age 16–17 | Age 22–32 | |
| Total sample | 91.2 | 85.1 | 84.3 | 58.0 |
| MZ males | 80.6 | 86.4 | 83.7 | 59.3 |
| MZ females | 96.9 | 88.1 | 88.1 | 62.3 |
| DZ males | 100.0 | 83.9 | 77.8 | 53.1 |
| DZ females | 100.0 | 86.0 | 87.2 | 58.0 |
| DZ opposite sex | 89.7 | 79.5 | 84.4 | 52.3 |
| % Current, light smokers (1–5 cigarettes smoked daily) | ||||
| Age 12–13 | Age 14–15 | Age 16–17 | Age 22–32 | |
| Total sample | 8.8 | 8.2 | 5.9 | 13.3 |
| MZ males | 19.4 | 6.8 | 7.8 | 15.7 |
| MZ females | 3.1 | 5.9 | 2.4 | 10.4 |
| DZ males | 0.0 | 9.7 | 7.4 | 12.5 |
| DZ females | 0.0 | 8.0 | 2.1 | 12.5 |
| DZ opposite sex | 10.3 | 12.3 | 7.1 | 16.5 |
| % Current, heavy smokers (5+ cigarettes smoked daily) | ||||
| Age 12–13 | Age 14–15 | Age 16–17 | Age 22–32 | |
| Total sample | 0.0 | 6.7 | 9.8 | 28.8 |
| MZ males | 0.0 | 6.8 | 8.5 | 25.0 |
| MZ females | 0.0 | 5.9 | 9.5 | 27.3 |
| DZ males | 0.0 | 6.5 | 14.8 | 34.4 |
| DZ females | 0.0 | 6.0 | 10.6 | 29.5 |
| DZ opposite sex | 0.0 | 8.2 | 8.4 | 31.3 |
Qualitative and Quantitative Sex Differences
Genetic analyses indicated that no significant qualitative or quantitative sex differences existed in the contribution of genetic or environmental factors to liability of smoking initiation and current quantity smoked, and in the relationship between smoking initiation and current quantity smoked for any of the age groups in this sample (see Table 2). More specifically, the same genes and environmental factors contributed to the liability of smoking initiation and current quantity smoked in males and females, and genetic and environmental contributions could be equated across sex across ages 14–15, 16–17, and 22–32. (Ages 12–13 were not included in these analyses due to inadequate sample size and ages 22–32 were combined to ensure adequate sample size for analyses.)
TABLE 2.
Model Fit Statistics from CCC Models
| Age 14–15 | EP | −2LL | df | AIC | diffLL | diffdf | p |
|---|---|---|---|---|---|---|---|
| Test for quantitative differences | |||||||
| 1. Different ACE + beta for males and females | 20 | 1,931.19 | 1,873 | −1,814.81 | — | — | — |
| 2. Equated parameters | 13 | 1,934.93 | 1,880 | −1,825.07 | 3.75 | 7 | 0.81 |
| CCC model fit comparisons | |||||||
| 2. Equated parameters across sex | 13 | 1,934.93 | 1,880 | −1,825.07 | — | — | — |
| 3. Model 2 + dropped beta | 12 | 1,935.67 | 1,881 | −1,826.33 | 0.74 | 1 | 0.39 |
| 4. Model 3 + dropped A from initiation | 11 | 1,947.25 | 1,882 | −1,816.75 | 12.32 | 2 | 0.00 |
| 5. Model 3 + dropped A from current quantity smoked | 11 | 1,936.07 | 1,882 | −1,827.93 | 1.14 | 2 | 0.57 |
| 6. Model 3 + dropped C from initiation | 11 | 1,938.78 | 1,882 | −1,825.22 | 3.84 | 2 | 0.15 |
| 7. Model 3 + dropped C from current quantity smoked | 11 | 1,939.71 | 1,882 | −1,824.29 | 4.78 | 2 | 0.09 |
| 8. Model 5 + dropped C from initiation | 10 | 1,939.18 | 1,883 | −1,826.82 | 4.24 | 3 | 0.24 |
| 9. Model 5 + dropped C from current quantity smoked | 10 | 1,977.13 | 1,883 | −1,788.87 | 42.2 | 3 | 0.00 |
| Age 16–17 | |||||||
| Test for quantitative differences | |||||||
| 1. Different ACE + beta for males and females | 20 | 2,096.96 | 1,858 | −1,619.04 | — | — | — |
| 2. Equated parameters | 13 | 2,099.69 | 1,865 | −1,630.31 | 2.72 | 7 | 0.91 |
| CCC model fit comparisons | |||||||
| 2. Equated parameters across sex | 13 | 2,099.69 | 1,865 | −1,630.31 | — | — | — |
| 3. Model 2 + dropped beta | 12 | 2,099.75 | 1,866 | −1,632.25 | 0.05 | 1 | 0.81 |
| 4. Model 3 + dropped A from initiation | 11 | 2,112.19 | 1,867 | −1,621.81 | 12.51 | 2 | 0.00 |
| 5. Model 3 + dropped A from current quantity smoked | 11 | 2,101.05 | 1,867 | −1,632.95 | 1.36 | 2 | 0.51 |
| 6. Model 3 + dropped C from initiation | 11 | 2,103.47 | 1,867 | −1,630.53 | 3.78 | 2 | 0.15 |
| 7. Model 3 + dropped C from current quantity smoked | 11 | 2,108.69 | 1,867 | −1,625.31 | 9.00 | 2 | 0.01 |
| 8. Model 3 + dropped C from initiation + A from current quantity smoked | 10 | 2,104.77 | 1,868 | −1,631.23 | 5.09 | 3 | 0.17 |
| Age 22–32 | |||||||
| Test for quantitative differences | |||||||
| 1. Different ACE + beta for males and females | 20 | 2,554.96 | 1,903 | −1,251.04 | — | — | — |
| 2. Equated parameters | 13 | 2,554.82 | 1,910 | −1,265.18 | −0.15 | 7 | 1.00 |
| CCC model fit comparisons | |||||||
| 2. Equated parameters across sex | 13 | 2,554.82 | 1,910 | −1,265.18 | — | — | — |
| 3. Model 2 + dropped beta | 12 | 2,559.40 | 1,911 | −1,262.60 | 4.59 | 1 | 0.03 |
| 4. Model 2 + dropped A from initiation | 12 | 2,567.20 | 1,911 | −1,254.80 | 12.39 | 1 | 0.00 |
| 5. Model 2 + dropped A from current quantity smoked | 12 | 2,558.10 | 1,911 | −1,263.90 | 3.29 | 1 | 0.07 |
| 6. Model 2 + dropped C from initiation | 12 | 2,554.82 | 1,911 | −1,267.18 | 0.00 | 1 | 1.00 |
| 7. Model 2 + dropped C from current quantity smoked | 12 | 2,555.28 | 1,911 | −1,266.72 | 0.46 | 1 | 0.50 |
| 8. Model 2 + dropped A from current quantity smoked and C from initiation | 11 | 2,558.37 | 1,912 | −1,265.63 | 3.55 | 2 | 0.17 |
| 9. Model 2 + dropped A from current quantity smoked and C from current quantity smoked | 11 | 2,574.40 | 1,912 | −1,249.60 | 19.58 | 2 | 0.00 |
| 10. Model 2 + dropped C from initiation and current quantity smoked | 11 | 2,555.28 | 1,912 | −1,268.72 | 0.46 | 2 | 0.79 |
Note: EP indicates the number of estimated parameters in the model.
Relationships between Smoking Initiation and Current Quantity Smoked
The relationship between smoking initiation and current quantity smoked could not be assessed for ages 12–13 years, due to inadequate sample size for the smoking quantity variable. Instead, univariate genetic analysis was conducted on the smoking initiation variable. The best fitting model for this age group did not include additive genetic factors, suggesting that common environmental (71.7%; 95% CI: 58.7%, 81.8%) and unique environmental factors (28.2%; 95% CI: 18.2%, 41.2%) best explained the variance in smoking initiation at age 12–13 years.
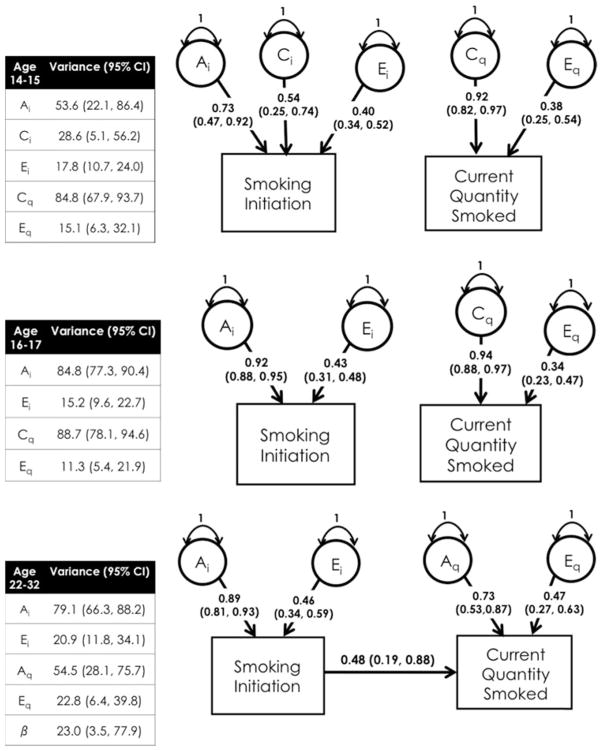
Across ages 14–15 and 16–17 years, dropping the beta parameter from the CCC model did not result in significantly worse model fit. This implied that smoking initiation and smoking quantity had independent liabilities at these age groups. The best fitting models were not the same across these age groups, however. For age 14–15 years, the best fitting models were an ACE model for smoking initiation and a CE model for current quantity smoked, suggesting that genetic (53.5%) and environmental factors (shared: 28.6%; unique: 17.8%) contributed to smoking initiation while environmental factors contributed to current quantity smoked (shared: 84.8%; unique: 15.2%), as measured by quantity smoked. For age 16–17 years, the best fitting model for smoking initiation was an AE model, while a CE model still fitted the data best for current quantity smoked, suggesting that genetic (84.8%) and unique (15.2%) environmental factors contributed to smoking initiation, while environmental factors (shared: 88.7%; unique: 11.3%) contributed to current quantity smoked (see Figure 1).
FIGURE 1.
Best fitting CCC models and variance component estimates.
For ages 22–32 years, the beta parameter between the initiation and current quantity smoked stages was significant, and the best fitting model was an AE model for both initiation and current quantity smoked. This suggested that smoking initiation and current quantity smoked shared liabilities to a moderate extent (β = 0.48) and was no longer independent, as with the earlier age groups. Additionally, genetic and unique environmental factors contributed to both smoking initiation and current quantity smoked, but shared environmental factors no longer exerted a signification impact on liability to smoking. Thus, of the genetic variance in liability to current quantity smoked, approximately 77.3% of the genetic variance was specific to current quantity smoked and 23.0% was shared with smoking initiation. In other words, mostly different genetic factors contributed to the liabilities of smoking initiation and current quantity smoked across adolescence, but in young adulthood, there was some overlap between the factors influencing initiation and current quantity smoked.
Discussion
No qualitative or quantitative differences were found between males and females regarding the genetic and environmental influences on individual differences in smoking initiation and current quantity smoked across adolescence into early adulthood, lending support for similar findings in other studies (Kendler et al., 1999; Koopmans et al., 1999). However, at age 22–32 years, when testing for qualitative sex differences, models constraining the genetic correlation to one, indicating the same genes influence smoking initiation and current quantity smoked in males and females, fitted the data only slightly better than models that allowed for the free estimation of the genetic correlation. Thus, it is possible that qualitative sex differences do exist in later adulthood and that we did not have the power to detect them in the current sample. This might explain why other studies utilizing adult samples have found qualitative sex differences in the genetic and environmental influences in smoking behavior (Heath et al., 1993; Kendler et al., 2005; Madden et al., 1999; Zavos et al., 2012).
Unfortunately, due to sample size constraints, we were unable to determine whether genetic or environmental factors contributed more significantly during the earliest ages of adolescence (ages 12–13 years). However, we did find that different factors contribute to smoking initiation and current quantity smoked across mid-adolescence into early adulthood. More specifically, smoking initiation and current quantity smoked seemed to have independent liabilities until adulthood, when liabilities were shared. Genetic, shared, and unique environmental factors were found to significantly contribute to smoking initiation during early adolescence (i.e., ages 14–15 years), but not during later adolescence (i.e., ages 16–17 years) or adulthood (i.e., ages 22–32 years), when genetic and unique environmental factors significantly contribute. Shared environmental influences may be more important for 14–15-year olds relative to older age groups because they experience greater limitations on the access to and availability of cigarettes. Although 14–15-year olds and 16–17-year olds experience the same legal age restriction on the purchasing of cigarettes, the 14–15-year olds might still have a harder time in gaining access to cigarettes among their peer groups if they have fewer friends who are of the legal age to buy cigarettes.
Additionally, genetic influences were not found to contribute significantly to smoking initiation until later adolescence into adulthood (beginning at age 14–15 years), much in the same way other studies suggest (Kendler et al., 2008; Koopmans et al., 1997; Slomowski et al., 2005). However, contrary to other findings, which found greater genetic influence on heavier/problem substance use, we found that genetic factors do not contribute significantly to the variance in current quantity smoked across all age groups until young adulthood (i.e., ages 22–32 years). Interestingly, it is also during this time that the liabilities of smoking initiation and quantity smoked are no longer independent of one another, but rather correlated. Again, this might be a function of access and availability to cigarettes. As access and availability of cigarettes increase, the expression of genetic predispositions towards increased smoking frequency and potential addiction may also increase, following initiation. Or, it could be the case that using a recent estimate of quantity smoked rather than an estimate from heaviest period of use is less stable and representative of adolescent youth relative to adults, and that our choice of measures for the analysis in this study could influence the estimate of the variance components.
Limitations and Strengths
Like all other studies, the present study has its limitations. Due to low prevalence of smoking behavior among early adolescents in this sample, the power of the current study was limited. This was apparent when we found that only a univariate genetic analysis could be conducted on the smoking initiation variable among 12–13-year olds as there were too many missing values for the current quantity smoked variable and consequently, a CCC model could not be fit. It is also possible that using self-reported data underestimated the prevalence for smoking behaviors, as a result of social desirability bias, which could have also influenced genetic analysis. Furthermore, this study is not generalizable to all populations, as the sample included only Caucasians.
Despite these shortcomings, the present study does include both males and females. It is also one of only a few studies investigating the relationship between smoking initiation and current quantity smoked within an adolescent sample and adds to the literature by investigating this relationship across various age groups. Future studies could include the use of measures related to smoking progression, other than current quantity smoked to investigate their effects on the relationship between smoking initiation and current quantity smoked. It would also be interesting to see if the same relationships are found among other adolescent datasets, using different populations than the one described in the present study and if these relationships are affected by the addition of environmental covariates, such as parental monitoring or peer influences.
Acknowledgments
This work was supported by the National Institutes of Health’s National Center for Advancing Translational Science (E.K.D., award number UL1TR000058) and the National Institutes of Health’s National Institute on Drug Abuse (E.K.D., H.H.M., project number 1R01DA025109-01A2: Developmental Genetic Epidemiology of Smoking).
References
- Agrawal A, Neale MC, Jacobson KC, Prescott CA, Kendler KS. Illicit drug use and abuse/dependence: Modeling of two-stage variables using the CCC approach. Addictive Behaviors. 2005;30:1043–1048. doi: 10.1016/j.addbeh.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Akaike H. Factor analysis and AIC. Psychometrika. 1987;52:317–322. [Google Scholar]
- Boker S, Neale M, Maes H, Wilde M, Spiegel M, Brick T, … Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broms U, Silventoinen K, Madden PA, Heath AC, Kaprio J. Genetic architecture of smoking behavior: A study of finnish adult twins. Twin Research and Human Genetics. 2006;9:64–72. doi: 10.1375/183242706776403046. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Current cigarette smoking among adults — United States, 2011. Morbidity and Mortality Weekly Report. 2012;61:889–894. [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention, & Office on Smoking and Health. Sustaining State Programs for Tobacco Control. Atlanta, GA: Author; 2008. State data highlights, 2006. [Google Scholar]
- Edwards KL, Austin MA, Jarvik GP. Evidence for genetic influences on smoking adult women twins. Clinical Genetics. 1995;47:236–244. doi: 10.1111/j.1399-0004.1995.tb04303.x. [DOI] [PubMed] [Google Scholar]
- Fowler T, Lifford K, Shelton K, Rice F, Thapar A, Neale MC, … van den Bree MBM. Exploring the relationship between genetic and environmental influences on initiation and progression of substance use. Addiction. 2007;102(3):413–422. doi: 10.1111/j.1360-0443.2006.01694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie NA, Neale MC, Kendler KS. Pathways to cannabis abuse: A multi-stage model from cannabis availability, cannabis initiation, and progression to abuse. Addiction. 2009;104:430–438. doi: 10.1111/j.1360-0443.2008.02456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath AC, Cates R, Martin NG, Meyer JM, Hewitt JK, Neale MC, Eaves LJ. Genetic contribution to risk of smoking initiation: Comparisons across birth cohorts and across cultures. Journal of Substance Abuse. 1993;5:221–246. doi: 10.1016/0899-3289(93)90065-j. [DOI] [PubMed] [Google Scholar]
- Heath AC, Kirk KM, Meyer JM, Martin NG. Genetic and social determinants of initiation and age at onset of smoking in Australian twins. Behavioral Genetics. 1999;29:395–407. doi: 10.1023/a:1021670703806. [DOI] [PubMed] [Google Scholar]
- Heath AC, Martin NG, Lynskey MT, Todorov AA, Madden PAF. Estimating two-stage models for genetic influences on alcohol, tobacco or drug use initiation and dependence vulnerability in twin and family data. Twin Research. 2002;5:113–124. doi: 10.1375/1369052022983. [DOI] [PubMed] [Google Scholar]
- Hewitt JK, Silberg JL, Rutter M, Simonoff E, Meyer JM, Maes HH, … Eaves LJ. Genetics and developmental psychopathology: 1. Phenotypic assessment in the Virginia twin study of adolescent behavioral development. Journal of Child Psychology and Psychiatry. 1997;38:943–963. doi: 10.1111/j.1469-7610.1997.tb01613.x. [DOI] [PubMed] [Google Scholar]
- Karp I, O’Loughlin J, Paradis G, Handley J, DiFranza J. Smoking trajectories of adolescent novice smokers in a longitudinal study of tobacco use. Annals of Epidemiology. 2005;15:445–452. doi: 10.1016/j.annepidem.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Gardner CO, Jacobson KC, Neale MC, Prescott CA. Genetic and environmental influences on illicit drug use and tobacco use across birth cohorts. Psychological Medicine. 2005;35:1349–1356. doi: 10.1017/S0033291705004964. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Neale MC, Sullivan P, Corey LA, Gardner CO, Prescott CA. A population-based twin study in women of smoking initiation and nicotine dependence. Psychological Medicine. 1999;29:299–308. doi: 10.1017/s0033291798008022. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Schmitt E, Aggen SH, Prescott CA. Genetic and environmental influences on alcohol, caffeine, cannabis, and nicotine use from early adolescence to middle adulthood. Archives of General Psychiatry. 2008;65:674–682. doi: 10.1001/archpsyc.65.6.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmans JR, Slutske WS, Heath A, Neale MC, Boomsma DI. The genetics of smoking initiation and quantity smoked in Dutch adolescent and young adult twins. Behavior Genetics. 1999;29:383–393. doi: 10.1023/a:1021618719735. [DOI] [PubMed] [Google Scholar]
- Koopmans JR, van Doornen LJ, Boomsma DI. Association between alcohol use and smoking in adolescent and young adult twins: A bivariate genetic analysis. Alcoholism Clinical and Experimental Research. 1997;21:537–546. [PubMed] [Google Scholar]
- Li MD, Cheng R, Ma JZ, Swan GE. A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction. 2003;98:23–31. doi: 10.1046/j.1360-0443.2003.00295.x. [DOI] [PubMed] [Google Scholar]
- Lyons M, Hitsman B, Xian H, Panizzon MS, Jerskey BA, Santangelo S, … Tsyang MT. A twin study of smoking, nicotine dependence, and major depression in men. Nicotine and Tobacco Research. 2008;10:97–108. doi: 10.1080/14622200701705332. [DOI] [PubMed] [Google Scholar]
- Madden PA, Heath AC, Pedersen NL, Kaprio J, Koskenvuo M, Martin NG. The genetics of smoking persistence in men and women: A multicultural study. Behavior Genetics. 1999;29:423–431. doi: 10.1023/a:1021674804714. [DOI] [PubMed] [Google Scholar]
- Maes HH, Sullivan PF, Bulik CM, Neale MC, Prescott CA, Eaves LJ. A twin study of genetic and environmental influences on tobacco initiation, regular tobacco use, and nicotine dependence. Psychological Medicine. 2004;34:1251–1261. doi: 10.1017/s0033291704002405. [DOI] [PubMed] [Google Scholar]
- Maes HH, Woodard CE, Murrelle L, Meyer JM, Silberg JL, Hewitt JK, … Eaves LJ. Tobacco, alcohol, and drug use in eight- to sixteen- year-old twins: The Virginia twin study of adolescent behavioral development. Journal of Studies on Alcohol and Drugs. 1999;60:293–305. doi: 10.15288/jsa.1999.60.293. [DOI] [PubMed] [Google Scholar]
- Meyer JM, Silberg JL, Simonoff E, Kendler KS, Hewitt JK. The Virginia twin-family study of adolescent and behavioral development: Assessing sample biases in demographic correlates of psychopathology. Psychological Medicine. 1996;26:1119–1133. doi: 10.1017/s0033291700035844. [DOI] [PubMed] [Google Scholar]
- Neale MC, Boker SM, Xie G, Maes HH. Statistical modeling. Richmond, VA: Virginia Commonwealth University, Department of Psychiatry; 2003. [Google Scholar]
- Neale MC, Harvey E, Maes HH, Sullivan PF, Kendler KS. Extensions to the modeling of initiation and progression; applications to substance use and abuse. Behavior Genetics. 2006;36:507–524. doi: 10.1007/s10519-006-9063-x. [DOI] [PubMed] [Google Scholar]
- Neyman J, Pearson ES. On the use and interpretation of certain test criteria for purposes of statistical inference. Biometrika. 1928;20:175–240. [Google Scholar]
- Slomowski C, Rende R, Novak S, Lloyd-Richardson E, Niaura R. Sibling effects on smoking in adolescence: Evidence for social influence from a genetically informative design. Addiction. 2005;100:430–438. doi: 10.1111/j.1360-0443.2004.00965.x. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. Results from the 2008 National Survey on Drug Use and Healt. Rockville, MD: Author; 2008. DHHS Publication No. SMA 09-4434 No. NS-DUH Series H-36. [Google Scholar]
- True WR, Heath AC, Scherrer JF, Waterman B, Goldberg J, Lin N, … Tsuang MT. Genetic and environmental contributions to smoking. Addiction. 1997;92:1277–1287. [PubMed] [Google Scholar]
- US Department of Health and Human Services. Preventing tobacco use among young people: Surgeon General’s Report. Washington, DC: Government Printing Office; 1994. No. DHHS publication 017-01-00491-0. [Google Scholar]
- Vink JM, Willemsen G, Boomsma DI. Heritability of smoking initiation and nicotine dependence. Behavior Genetics. 2005;35:397–406. doi: 10.1007/s10519-004-1327-8. [DOI] [PubMed] [Google Scholar]
- Vuong QH. Likelihood ratio tests for model selection and non-nested hypotheses. Econometrica. 1989;57:307–333. [Google Scholar]
- Williams LHHPJ. Parsimony-based fit indices for multiple-indicator models: Do they work? Structural Equation Modeling. 1994;1:161–189. [Google Scholar]
- Zavos HM, Kovas Y, Ball HA, Ball D, Siribaddana SH, Glozier N, … Rijsdijk FV. Genetic and environmental etiologiy of nicotine use in Sri Lankan male twins. Behavior Genetics. 2012;42:798–807. doi: 10.1007/s10519-012-9544-z. [DOI] [PMC free article] [PubMed] [Google Scholar]