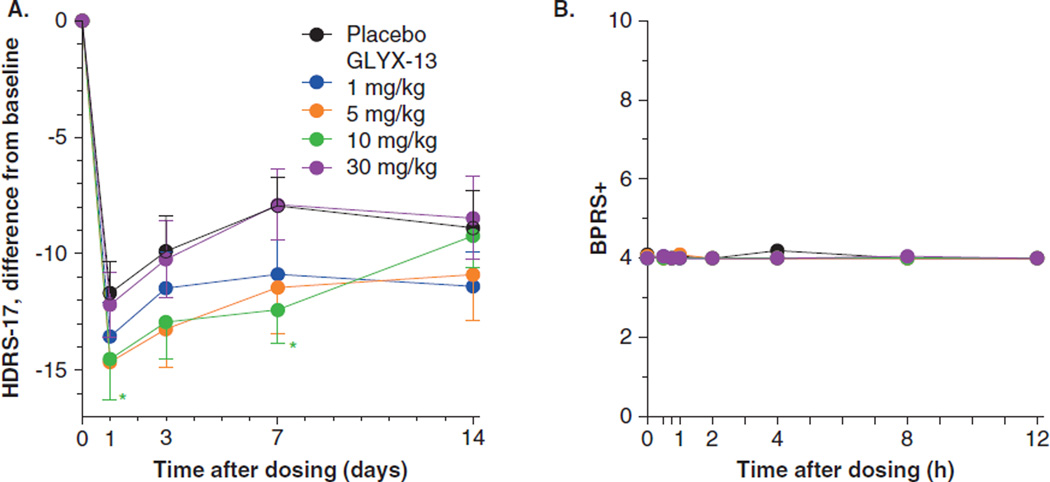
Figure 5. GLYX-13 reduces depression scores assessed as HDRS-17 score and does not cause psychotomimetic side effects assessed as BPRS+ scores.
(A) HDRS-17 scores are presented as difference from baseline. Mean ± SEM. Baseline scores were 26.1 (n = 33) placebo, and 26.1 (n = 25), 25.2 (n = 20), 25.1 (n = 17), and 24.6 (n = 21) for GLYX-13, 1, 5, 10, and 30 mg/kg, respectively. *p < 0.05 for 10 mg/kg compared to placebo at that time point. *p < 0.05 ANCOVA for 5 and 10 mg/kg compared to placebo. GLYX-13 reduces depressive symptoms assessed as Bech-6 scores. (B) GLYX-13 does not cause psychotomimetic side effects assessed as BPRS+ scores.
Mean + SEM, SEM were all 0.02 or less. Placebo, n = 33, GLYX-13 1 mg/kg, n = 25, 5 mg/kg, n = 20, 10 mg/kg, n = 17, 30 mg/kg, n = 21.