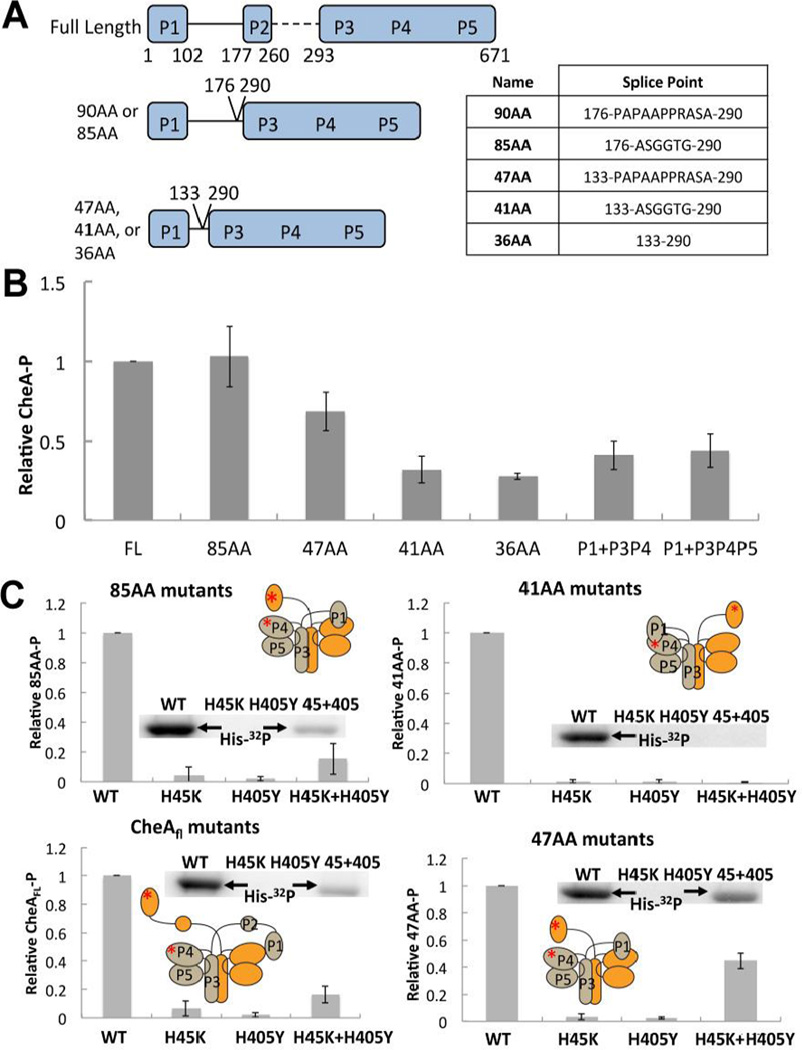
Figure 1. ΔP2 variants and their autophosphorylation activity.
(A) Cartoon depiction of ΔP2 variants of Thermotoga maritima CheA with domain boundaries and splice points indicated, nomenclature is based on the linker length (see text). Table at right shows residues inserted between splice points marked on left. (B) Relative autophosphorylation activity of T. maritima CheAFL, ΔP2 variants, or separated domains. Levels of P1-P were determined after 6 min of exposure to γ-32P-ATP at 25 °C. All proteins are at 10 µM subunit concentration. (C) Rescue of autophosphorylation activity of CheAFL, 85AA, 47AA, and 41AA active site mutants (P1:H45K or P4:H405Y) by subunit exchange. A phosphorimage of the gel assays shows P1-P production. Autophosphorylation is only possible in the trans subunit reaction of a heterodimer that contains a WT P1 subunit and a WT P4 subunit (45 + 405) A cartoon depiction of trans or cis autophosphorylation for each variant is inset with a red asterisk depicting the point mutations that abrogate function of the P1 and P4 domains in the mixed dimer. All proteins are at a subunit concentration of 10 µM and were heated overnight at 55 °C to allow for subunit exchange prior to γ-32P-ATP being added for 6 minutes at 25 °C.