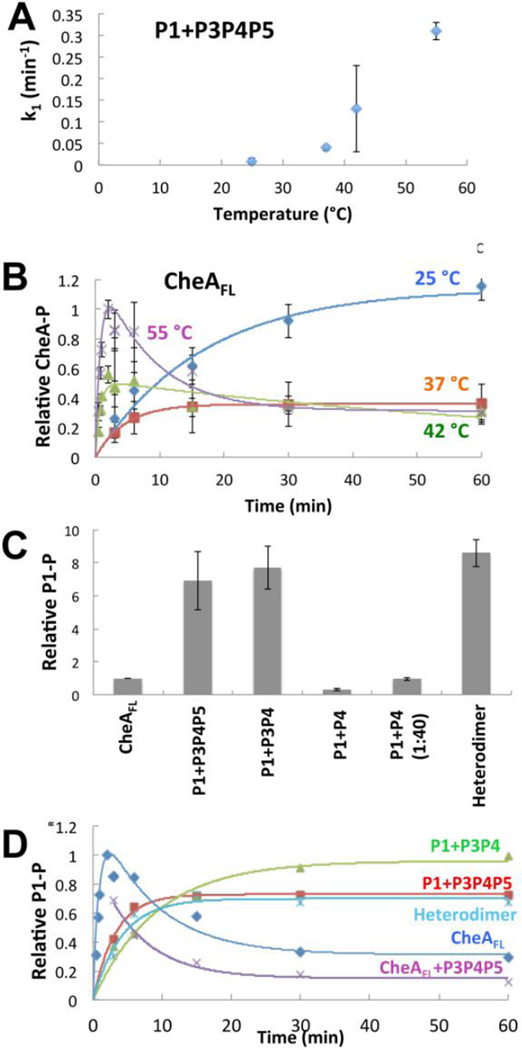
Figure 4. Autophosphorylation activity for T. maritima CheAFL and separated domains.
(A) Rate constants for accumulation of P1-P by P1+P3P4P5 as a function of temperature. Higher temperatures increase P1 phosphorylation rates, which fit well to first order kinetics. (B) Time courses for autophosphorylation of CheAFL as a function of temperature. At 42 °C CheAFL begins to show biphasic behavior in which P1-P peaks at early times and then diminishes to a lower constant level. This behavior accentuates at 55 °C. All proteins are at 10 µM. Data were fit to first order kinetics for low temperatures and to the kinetic described in the Materials and Methods for 42 °C and 55 °C. (C) Production of phosphorylated CheAFL and separated domains (all at 10 µM) after 60 min at 55 °C. Separation of P1 from P4 increases activity, but only when the P3 domain is fused to P4. Even in high molar excess (40:1) P4 only weakly phosphorylates P1. In contrast to CheAFL, CheAFL:P3P4P5 heterodimers (Heterodimer) containing only one P1 domain show efficient P1 phosphorylation. (D) Time courses for P1-P formation for CheA (10 µM) and separated domains at 55 °C. The separated domains and the CheAFL:P3P4P5 heterodimer accumulate P1-P, whereas P1-P diminishes over time with CheAFL with or without added (but not exchanged) P3P4P5. Error bars of similar magnitude as shown in (A), but not shown for clarity. Each point is an average from 3–5 individual experiments.