Abstract
Glucocorticoid hormones play a pivotal role in the response to stressful challenges. The surge in glucocorticoid hormone secretion after stress needs to be tightly controlled with characteristics like peak height, curvature and duration depending on the nature and severity of the challenge. This is important as chronic hyper- or hypo-responses are detrimental to health due to increasing the risk for developing a stress-related mental disorder. Proper glucocorticoid responses to stress are critical for adaptation. Therefore, the tight control of baseline and stress-evoked glucocorticoid secretion are important constituents of an organism's resilience. Here, we address a number of mechanisms that illustrate the multitude and complexity of measures safeguarding the control of glucocorticoid function. These mechanisms include the control of mineralocorticoid (MR) and glucocorticoid receptor (GR) occupancy and concentration, the dynamic control of free glucocorticoid hormone availability by corticosteroid-binding globulin (CBG), and the control exerted by glucocorticoids at the signaling, epigenetic and genomic level on gene transcriptional responses to stress. We review the beneficial effects of regular exercise on HPA axis and sleep physiology, and cognitive and anxiety-related behavior. Furthermore, we describe that, possibly through changes in the GABAergic system, exercise reduces the impact of stress on a signaling pathway specifically in the dentate gyrus that is strongly implicated in the behavioral response to that stressor. These observations underline the impact of life style on stress resilience. Finally, we address how single nucleotide polymorphisms (SNPs) affecting glucocorticoid action can compromise stress resilience, which becomes most apparent under conditions of childhood abuse.
Keywords: Exercise, HPA axis, Glucocorticoid receptor, Mineralocorticoid receptor, CBG, PTSD
1. Introduction
Glucocorticoid hormones play a fundamental role in the adaptation of an organism to stressful events in its life. Research over the past >60 years has shown that glucocorticoid hormone actions at the molecular and cellular level are highly complex with multiple long-term consequences for physiology and behavior (De Kloet and Reul, 1987, De Kloet et al., 1998, De Kloet et al., 2005, McEwen, 2012a, McEwen, 2012b). Not surprisingly, research has provided ample evidence that chronic hyper- as well as hypo-secretion of glucocorticoid hormones is involved in the development of a range of metabolic, immune, endocrine and neuro-psychiatric disorders. The psychiatric diseases include stress-related disorders like major depression and anxiety disorders (e.g. post-traumatic stress disorder (PTSD)). During the past 15 years this idea has been supported by evidence that individual differences exist in the vulnerability of developing a major depressive or anxiety disorder during the course of life (Zannas and Binder, 2014). It appears that certain genetic traits, e.g. SNPs in the glucocorticoid receptor (GR; Nr3c1) associated chaperone Fkbp5 (FK506-binding protein 51) gene, in combination with traumatic (early) life events can dramatically increase the likelihood of precipitating psychiatric disease (Klengel and Binder, 2013a, Klengel and Binder, 2013b). Conversely, mechanisms are in place to maintain or increase resilience of the organism to stress and prevent the development of maladaptive responses and disease. Evidence has been accumulating that a physically active life style (exercise) is beneficial in strengthening resilience to stress (Reul and Droste, 2005). Indeed, it has been shown that long-term voluntary exercise in rodents such as rats and mice results in changes in HPA axis control, sleep physiology, and anxiety-related behavior (Droste et al., 2003, Lancel et al., 2003, Binder et al., 2004a).
In this article we will review the role of glucocorticoid hormones in resilience. We define resilience as an individual's ability to effectively adapt to stress and adversity, resulting in the prevention of physical and/or psychological disease. We will address recently discovered mechanisms dynamically regulating the biological availability of glucocorticoid hormones. Novel insights into the role of this hormone in epigenetic mechanisms associated with gene transcriptional and behavioral responses to stress will be described. We will review evidence that increasing physical activity in one's life style enhances stress resilience. Finally, we will highlight how early life trauma can affect life-long glucocorticoid action.
2. Glucocorticoid action via MRs and GRs: early findings and concepts
It has been almost 30 years ago since the binding properties of the natural glucocorticoid hormone to receptors in rodent brain have been described (Reul and De Kloet, 1985). Reul and de Kloet discovered that corticosterone binds to two types of receptors, the mineralocorticoid receptor (MR; also termed ‘Type 1’ in the early days) and the GR (also termed ‘Type 2’), in the high-speed soluble fraction (‘cytosol’) of hippocampus homogenates (Reul and De Kloet, 1985). Highest levels of MRs are typically found in dentate gyrus, CA2 and CA1 of the hippocampus, lateral septum and central amygdala whereas GRs are found throughout the brain with high concentrations in the hippocampus, neocortex and hypothalamic nuclei such as the paraventricular nucleus (PVN) and supraoptic nucleus (Reul and De Kloet, 1985, Reul and De Kloet, 1986, Reul et al., 1987, Kiss et al., 1988). This localization pattern was confirmed after the receptor had been cloned (Hollenberg et al., 1985, Arriza et al., 1987) and in situ hybridization and immunohistochemical studies had been performed (Fuxe et al., 1985a, Fuxe et al., 1985b, Herman et al., 1989a, Van Eekelen et al., 1988, Reul et al., 2000, Gesing et al., 2001). A similar distribution of MRs and GRs as found in the rat and mouse brain was found in the dog brain albeit that the brain localization of MRs is more widespread in this species than in rodents (Reul et al., 1990). Scatchard and Woolf plot analyses showed that MRs bind corticosterone with an extraordinarily high affinity (0.1–0.5 nM) whereas GRs bind the natural hormone with a lower affinity (2.5–5 nM) (Reul and De Kloet, 1985, Reul et al., 1987). As a result of this stark difference in binding affinity, subsequent studies presented a marked difference between MRs and GRs in the degree of occupancy by endogenous glucocorticoid hormone under baseline and stress conditions (Reul and De Kloet, 1985). Under baseline early morning conditions, MRs already showed a high occupancy whereas GRs were hardly occupied. In contrast, at the circadian peak and even more strongly after stress both receptor types showed a high degree of occupancy by endogenous hormone (Reul and De Kloet, 1985).
At the time, the concept of a glucocorticoid-binding receptor, i.e. MR, which under any physiological conditions is highly occupied with endogenous hormone, was rather controversial. As usually receptor signaling is thought to depend on the degree of receptor occupancy by ligand whose concentration is determined by the physiological condition at hand; a receptor like MR that is always substantially occupied would defeat this purpose. Based on the remarkably distinct properties of MRs and GRs in the hippocampus in conjunction with neuroendocrine and other observations, De Kloet and Reul (De Kloet and Reul, 1987, Reul and De Kloet, 1985) developed a concept that amalgamated these properties in a unifying model on glucocorticoid action in this limbic brain structure. In this concept, hippocampal MRs confer tonic inhibitory influences of circulating glucocorticoids that serve to restrain baseline HPA axis activity (De Kloet and Reul, 1987, Reul and De Kloet, 1985). Neuroanatomical, pharmacological and lesion studies indeed showed that the hippocampus exerts a tonic inhibitory influence on the activity of PVN neurons in the hypothalamus, driven trans-synaptically through distinct populations of GABA-ergic neurons in the bed nucleus of the stria terminalis (BNST; De Kloet and Reul, 1987, De Kloet et al., 2005, Herman et al., 1989b, Herman and Cullinan, 1997, Herman et al., 2003). In accordance with their responsiveness to elevated glucocorticoid levels and the mediation of the HPA axis-suppressing effects of synthetic glucocorticoids like dexamethasone, GRs are considered to be responsible for the negative feedback action of glucocorticoid hormones (De Kloet and Reul, 1987, Reul and De Kloet, 1985). They do so mainly at the anterior pituitary and PVN level but effects via GRs located in the hippocampus, prefrontal cortex, amygdala and other parts of the brain cannot be excluded (De Kloet and Reul, 1987, De Kloet et al., 2005, Reul and De Kloet, 1985, Herman et al., 2003). The hippocampal MRs and GRs also play distinct roles in the control of sympathetic outflow and in behavioral responses to stressful events (De Kloet et al., 2005). Potent MR- and/or GR-mediated effects of glucocorticoid hormones have been shown in various hippocampus-associated behavioral tests such as the forced swim test, Morris water maze learning and contextual fear conditioning (Jefferys et al., 1983, Veldhuis et al., 1985, Bilang-Bleuel et al., 2005, Gutierrez-Mecinas et al., 2011, Mifsud et al., 2011, Trollope et al., 2012, Reul, 2014, Oitzl et al., 2001, Beylin and Shors, 2003, Zhou et al., 2010). At the cellular level, distinct electrophysiological effects of glucocorticoid hormones via MRs and GRs on hippocampal neurons have been described (Joëls and De Kloet, 1992, Pavlides et al., 1993, Joëls et al., 2009).
In this manner, the dual glucocorticoid-binding receptor system regulates the physiological (including endocrine and autonomic) responses and behavioral responses under baseline and stress conditions thereby maintaining homeostasis and facilitating long-term adaptation, together safeguarding resilience of the organism. The mechanisms underlying resilience are complex and multifaceted. Furthermore, the capacity to cope with and adapt to adverse events is influenced by life style, genetic vulnerability and early life factors. Presently, we are only beginning to understand these mechanisms. Here, we describe several findings that portray the importance and complexity of the role of MRs and GRs in resilience. This is not a complete listing as this would go beyond the scope of this review. The described findings address the diversity and complexity of the mechanisms involved and are regarded as particularly important for future developments.
3. Dynamic control of hippocampal MRs as an instrument of resilience
The high degree of occupancy of hippocampal MRs under any physiological circumstance was a controversial finding because how would such a receptor system be able to adjust signaling to different circumstances? The answer turned out to be: by dynamically adjusting the concentration of receptor molecules in neurons. Serendipitously, we observed that acute stressful challenges that engage the hippocampus like forced swimming and novelty exposure resulted in a significant increase in the concentration of MRs, but not GRs, in the hippocampus of rats (Gesing et al., 2001). The rise was transient and occurred between 8 and 24 h after the challenge. Remarkably, this effect of stress turned out to be mediated by corticotropin-releasing factor (CRF). Intracerebroventricular injection of the neuropeptide resulted in a rise in hippocampal MRs whereas pre-treatment with a CRF receptor antagonist blocked the effect of forced swimming on MRs. Interestingly, CRF injection was ineffective in adrenalectomized rats; concomitant MR occupancy appeared to be a necessity for CRF to produce an increase in hippocampal MR levels indicating a permissive role of the receptor in this process (Gesing et al., 2001). The observation that CRF mimicked the stress effect on MRs suggested the involvement of CRF1 receptors (Reul and Holsboer, 2002). It was indeed found that forced swimming failed to raise hippocampal MR mRNA concentrations in mice carrying a gene deletion of CRF1 receptor (Muller et al., 2003). The effect of CRF on MRs was a remarkable novel finding as we are dealing with one of the principal mediators of acute stress response in the brain, i.e. CRF, acting upon a main stress controlling instrument, i.e. MR. Changes in hippocampal serotonin levels may also be involved in the effect of forced swimming on MR as this stressor has been shown to increase hippocampal serotonin; an effect which was mimicked by central CRF administration (Linthorst et al., 2002, Linthorst et al., 2008). Serotonin has been shown to be involved in MR and GR regulation (Seckl and Fink, 1991, Vedder et al., 1993). The rise in MRs after stress proved to have functional consequences for the control of baseline HPA axis activity. Administration of the selective MR antagonist RU28318, 24 h after swim stress, i.e. at the time point when MRs are increased, resulted in a substantially larger rise in baseline HPA axis activity in rats which had been forced to swim 24 h earlier than in unstressed control animals (Gesing et al., 2001). This indicates that, concomitantly with the rise in receptor concentration, the MR-mediated inhibitory control of the HPA axis had increased after stress. Thus, the stress-CRF-MR mechanism appears to participate in safeguarding normal HPA axis activity with the aim to prevent the development of glucocorticoid hyper-secretion with its associated adverse effects on the organism. Therefore, this mechanism may be important to maintain resilience to stress.
In aging and depressed subjects this mechanism may be failing. Many years ago it was found that hippocampal MR levels are significantly decreased and baseline and stress-induced HPA axis activity is increased in aged rats and dogs (Reul et al., 1988, Reul et al., 1991, Rothuizen et al., 1993). In some post-mortem studies on people with a history of major depressive illness, increased levels of CRF concentrations in cerebrospinal fluid and decreased levels of CRF-binding capacity has been shown (Nemeroff et al., 1984, Nemeroff et al., 1988, Swaab et al., 2005). In Alzheimer's disease increase activation of central CRF neurons has been reported as well (Swaab et al., 2005). Chronically elevated CRF concentrations have vast implications for central neurotransmission (e.g. serotonin) as well as for the control of system physiology and behavior (e.g. body temperature, immune system regulation, circadian behavioral activity) (Linthorst et al., 1997, Labeur et al., 1995). A recent publication reported on the role of the CRF1 receptor in the effects of chronic stress on Alzheimer's disease related molecules in the hippocampus and behavior (Carroll et al., 2011). Thus, in aged subjects, CRF/CRF1 receptor associated mechanisms to maintain hippocampal MR function seem to be failing but more research is required to support this notion. Interestingly, hippocampal MR levels are particularly sensitive to neurotrophic factors and antidepressant drug treatment (Reul et al., 1988, Reul et al., 1993, Reul et al., 1994, De Kloet et al., 1987), however, how these findings relate to changes in the CRF-MR system is currently unknown.
4. CBG – a novel role for the control of stress-induced glucocorticoid hormone concentrations
For many years, corticosteroid-binding globulin (CBG) has been thought to be simply just a transport protein for endogenous glucocorticoid hormone. The levels of CBG were thought to be quite stable in the circulation, and depending on the activity state of the HPA axis, allowing approximately between 1 and 10% of circulating plasma glucocorticoid hormone to be ‘free’, i.e. unbound, and thus capable to penetrate tissues and bind to glucocorticoid-binding receptors. However, in 2008 the HPA axis field was about to receive a stir. The prelude to this started in the early 1990s when we were the first to start using in vivo microdialysis in freely behaving rats and mice to study free corticosterone levels in the brain under various physiological conditions (Linthorst et al., 1994, Linthorst et al., 1995). It proved to be a powerful technique allowing monitoring of free glucocorticoid hormone levels in the extracellular space of different brain regions, like the hippocampus, with a high time resolution over several days without the need to interfere with the animal (Linthorst and Reul, 2008). Comparing various studies over a number of years, we noted a discrepancy between the time courses of the free glucocorticoid hormone response and the total plasma hormone responses after stress. The free glucocorticoid response after stressors like forced swimming (15 min, 25 C water) peaked at approximately 1 h after the start of the stressor (Droste et al., 2009b) whereas the total plasma hormone response was already at its highest level at 30 min (Bilang-Bleuel et al., 2002). In a study which directly compared the plasma glucocorticoid response and free hormone response in the hippocampus after forced swimming using blood sampling and microdialysis, respectively, a time delay between the two responses of 20–25 min was indeed confirmed (Droste et al., 2008). The delay was not due to a tardy penetration of the hormone into the extracellular space of the brain because parallel microdialysis of the brain, the blood and the subcutaneous tissue showed highly similar free glucocorticoid levels under baseline, circadian conditions (Qian et al., 2012) and in response to stress (Qian et al., 2011) in these different compartments. The delayed free corticosterone response to stress was further assessed using different stress paradigms including forced swimming, restraint and novelty stress. We discovered that subjecting rats to a stressful situation resulted in a rapid rise in circulating CBG concentrations in the blood (Qian et al., 2011). The extent of the rise depended on the magnitude of the glucocorticoid hormone response evoked by the stressor. Hence, strong stressors like forced swimming and restraint produced substantially higher rises in plasma CBG than a mild stressor like novelty stress that led to a negligible increase in the binding protein (Qian et al., 2011). As mentioned, the rise in plasma CBG has a rapid onset reaching maximal levels within 15–30 min after the start of forced swimming and returning to baseline values between 2 and 8 h later. Thus, the rise in circulating CBG evolving in parallel with the rise in total plasma corticosterone and sequestering these glucocorticoid hormone molecules is the reason for the delay of approximately 25 min in the rise of free hormone levels (Droste et al., 2008, Qian et al., 2011). The logical question came up: where is the significant amount of CBG molecules coming from? As the surge in plasma CBG levels was so rapid, de novo synthesis was highly unlikely. Nevertheless, we embarked to investigate the prime site of CBG synthesis, which is the liver (Hammond, 1990, Hammond et al., 1991). Immunohistochemical analysis revealed that liver cells store substantial amounts of CBG. Remarkably, within 30 min after forced swimming virtually all CBG had disappeared from the organ, presumably into the circulation (Qian et al., 2011). Twenty-four hours later CBG content in the liver had returned to its normal levels (Qian et al., 2011); whether this is due to re-synthesis or retrieval from the circulation is presently unknown.
This recent work identifies CBG as a principal regulatory factor in glucocorticoid homeostasis and function. It plays a defining role in not only the degree to which tissue is exposed to glucocorticoid hormone but also in determining the exact timing during which this is happening. Timing has been shown to be an important factor in glucocorticoid action (Munck et al., 1984, Wiegers and Reul, 1998). Studies in CBG knockout mice have suggested as well that CBG plays a complex role in the regulation of glucocorticoid hormones (Petersen et al., 2006, Richard et al., 2010). Currently, however, it is unknown whether compensatory mechanisms may have contributed to the phenotypic findings in animals with a life-long CBG deficiency. Therefore, if mutant mouse models are the chosen route of investigation, forthcoming studies should be directed at inducible and tissue-specific CBG knockout mouse models.
These novel insights underscore the great significance of CBG for stress resilience. Future research should elucidate the signaling, epigenetic and gene transcriptional mechanisms governing the secretion/release and synthesis of this very interesting binding protein.
5. Glucocorticoid regulation of epigenetic mechanisms controlling gene transcription and behavior
It has been known for many years that glucocorticoid hormones have a potent influence on behavior. These effects have been shown repeatedly in various behavioral paradigms such as the forced swim test, Morris water maze learning and contextual fear conditioning (Jefferys et al., 1983, Veldhuis et al., 1985, Gutierrez-Mecinas et al., 2011, Beylin and Shors, 2003, Zhou et al., 2010, Cordero and Sandi, 1998, Oitzl and De Kloet, 1992, Sandi et al., 1997). In the learning phase of these paradigms, glucocorticoid hormones are secreted in response to the stress associated with being submitted (involuntarily) into a container filled with water (forced swim test, Morris water maze) or into a shock box (fear conditioning). The elevated concentrations of hormone (predominantly via GRs) facilitate the consolidation of memories specifically associated with the adverse event. It is important to underline that glucocorticoids only exert this role if their concentrations rise within the context of the adverse event. If levels rise, for instance as a result of a stressor (e.g. electric foot shock(s)), before the event, then glucocorticoids have been shown to impair learning and memory processes (De Kloet et al., 2005, McEwen, 2001). Also chronic stress, leading to persistently elevated glucocorticoid hormones, has been reported to impair cognitive processes (De Kloet et al., 2005, McEwen, 2001). Due to these distinct roles of glucocorticoids in learning and memory there is often confusion in the scientific literature (and in the media!) about the effects of stress or glucocorticoids on learning and memory. Here we will focus on the role of glucocorticoids during the consolidation phase of acute adverse events, thus when the action of these hormones helps to make memories of the event thereby supporting behavioral adaptation and resilience of the organism.
Although a role of glucocorticoids on behavior has been known for many years, only fairly recently some insight was revealed into the mechanism of action of these hormones (Gutierrez-Mecinas et al., 2011). Most progress in this respect has been made using the forced swim test but the mechanism uncovered is likely transposable to the Morris water maze and contextual fear conditioning paradigms (Reul, 2014, Reul and Chandramohan, 2007). In the forced swim test, rats or mice are placed in a beaker containing water (usually at 25 C; duration 15 min (mice: 10 min)) from which they cannot escape. The animal will try to escape but quickly finds out that this is impossible and adopts a so-called floating or immobility position to conserve energy (De Pablo et al., 1989, Korte, 2001). If the animal is re-introduced to the water 24 h later, after initial brief attempts to escape it will predominantly show immobility behavior and to a much greater extent than in the initial test. Even if the animal is re-tested 4 weeks after the initial test it will show this behavioral immobility response (Gutierrez-Mecinas et al., 2011). Thus, based on memories the animal has formed after the initial forced swim session, it quickly decides in the favor of the adaptive behavioral immobility strategy to increase its chances for survival (Reul, 2014, Reul and Chandramohan, 2007).
Studies since the early 1980s have shown that the behavioral immobility response in the re-test is critically dependent of glucocorticoid hormone action via GRs during the hours after the initial test. Adrenalectomized rats are severely impaired in this behavioral response (Jefferys et al., 1983, Veldhuis et al., 1985, Mitchell and Meaney, 1991). Behavior in these animals can be rescued if given a GR agonist like corticosterone or dexamethasone at the time of the initial test (Jefferys et al., 1983, Veldhuis et al., 1985, Mitchell and Meaney, 1991). Rats treated with a GR antagonist like RU38486 or ORG34517 show normal behavior in the initial test but an impaired immobility response in the re-test (Gutierrez-Mecinas et al., 2011, De Kloet et al., 1988). More details about the pharmacology of this behavioral test were addressed recently elsewhere (Reul, 2014). As mentioned, until recently the mechanism of action of glucocorticoid hormone in this test was completely unknown. The neuroanatomical site of hormone action however has been known since 1988 when de Kloet and colleagues reported that micro-injection of GR antagonist specifically into the dentate gyrus of the hippocampus impaired the behavioral immobility response (De Kloet et al., 1988). We recently elucidated how glucocorticoids via GRs are implemented in this process. We discovered that in addition to GRs, dentate gyrus N-methyl d-aspartate (NMDA) receptors activating the mitogen-activated protein kinase (MAPK) pathway are also involved (Gutierrez-Mecinas et al., 2011, Chandramohan et al., 2008). Forced swimming results, via a sparse activation of NMDA receptors, in the specific phosphorylation of the MAPKs extracellular signal-regulated kinase 1 and 2 (ERK1/2; also termed p42/44-MAPK). pERK1/2 subsequently phosphorylates the two downstream chromatin-modifying kinases mitogen- and stress-activated kinases 1 and 2 (MSK1/2) and ets-like kinase 1 and 2 (Elk1/2). pMSK1/2 was shown to phosphorylate histone H3 at serine10 (S10) whereas pElk1/2, via recruitment of histone acetyl-transferases (HATs) like p300, evoke the acetylation of lysine14 (K14), thus forming the combinatorial epigenetic marks H3S10p-K14ac (Gutierrez-Mecinas et al., 2011, Chandramohan et al., 2008). The formation of these epigenetic marks in the promoter region of intermediate-early genes (IEGs) like c-Fos and Egr-1 (also called NGFI-A or Zif268) facilitated the induction of these genes (Gutierrez-Mecinas et al., 2011). Injection of a GR-occupying dose of corticosterone was ineffective in terms of H3S10p-K14ac formation and IEG induction (Chandramohan et al., 2007), indicating indeed that, in addition to GR, activation of the NMDA receptor pathway is required. Previous work has shown that the H3S10p-K14ac mark is particularly involved in the opening of silent genes, possibly through chromatin remodeling, making them accessible for transcription (Cheung et al., 2000a, Cheung et al., 2000b, Nowak and Corces, 2000). The interesting notion may be extracted that these dual histone marks tag genes that were silent before the animal was stressed. Neuroanatomically it is of interest to note that the activation of this signaling and epigenetic pathway leading to IEG induction was specifically observed in sparsely distributed mature granule neurons located in the dorsal blade of the dentate gyrus of rats and mice (Bilang-Bleuel et al., 2005, Gutierrez-Mecinas et al., 2011, Chandramohan et al., 2007, Chandramohan et al., 2008). Similar characteristic biochemical (pathway) and neuroanatomical changes in dentate gyrus neurons were observed after Morris water maze training, contextual fear conditioning and novelty exposure (Chandramohan et al., 2007, Chwang et al., 2007; Carter S.D., Mifsud K.R. & Reul J.M.H.M., unpublished observations). These observations are commensurate with the normal physiology of the dentate gyrus, i.e. the NMDA receptor-mediated sparse activation of mature dentate neurons after a challenge. Therefore, we have previously hypothesized (Reul, 2014, Reul et al., 2009) that the observed signaling and epigenetic changes are taking place in neurons involved in a process called pattern separation (Treves and Rolls, 1994, Rolls and Kesner, 2006); a physiological process which is thought to be required for sensory information processing in the dentate gyrus and memory formation.
Other researchers and we have indeed shown that various constituents of the NMDA/ERK1/2/MSK1/2–Elk-1 pathway are required for memory formation in the Morris water maze, contextual fear conditioning and the forced swim test (Gutierrez-Mecinas et al., 2011, Chandramohan et al., 2008, Chwang et al., 2007). Several research groups have shown that the NMDA receptor and the MAPK pathway are critical for learning in these tests (Chandramohan et al., 2008, Chwang et al., 2007). David Sweatt and colleagues reported that MSK1 gene deleted mice are impaired in the Morris water maze and contextual fear conditioning paradigms (Chwang et al., 2007). We reported that the behavioral immobility response in the forced swim test is gravely disturbed in MSK1/2 double gene knock-out mice (Chandramohan et al., 2008). Furthermore, in a series of pharmacological and neuroanatomical studies we found that inhibition of any step of the NMDA/ERK1/2/MSK1/2–Elk-1 pathway in dentate gyrus neurons resulted in a significant reduction in the IEG response and an impaired behavioral immobility response (Gutierrez-Mecinas et al., 2011, Chandramohan et al., 2008).
The activation of the previously described signaling and epigenetic pathway along with GRs at dentate gyrus neurons is involved in the consolidation of the behavioral immobility response. The question arose how these two pathways are involved in establishing this behavioral response. An important lead was provided by the observation that administration of a GR antagonist before forced swimming resulted in a strongly diminished c-Fos and Egr-1 response in dentate neurons (Gutierrez-Mecinas et al., 2011). Moreover, the antagonist also inhibited the stress-induced responses in pMSK1/2 and pElk1/2 in these neurons but did not affect the pERK1/2 response (Gutierrez-Mecinas et al., 2011). Based on these observations we postulated that in the forced swim situation, activated GRs, through interaction with pERK1/2, facilitate the phosphorylation of MSK1/2 and Elk-1, which was indeed confirmed by co-immuno-precipitation experiments (Gutierrez-Mecinas et al., 2011). This novel, non-genomic mode of GR action is relatively fast as pERK1/2, pMSK1/2 and pElk1 peak at approximately 15 min after start of forced swimming. Since these molecules also play a role in Morris water maze learning and fear conditioning this mechanism may play a role in these paradigms as well but this needs to be confirmed. This was the first time a functional interaction between GRs, pERK1/2, pMSK1/2 and pElk1 has been observed. Previously, Miguel Beato and colleagues reported a crucial role of the interaction of the progesterone receptor with ERK1/2 and MSK1/2 in the phosphorylation of S10 in histone H3 in cells in vitro (Vicent et al., 2006). Thus, in dentate gyrus neurons, after a challenge the convergence of two signaling pathways is crucial for the proper activation of chromatin-modifying enzymes to subsequently elicit epigenetic changes and induction of gene transcription. In this manner, enhanced glucocorticoid hormone secretion as a result of the stressful challenge facilitates a now well-defined molecular mechanism that underlies the consolidation of appropriate cognitive behavioral responses to the challenge, which are adaptive and beneficial for the organism (Reul, 2014, Reul and Chandramohan, 2007, Reul et al., 2009). Therefore, this novel glucocorticoid mechanism participates in the maintenance of resilience.
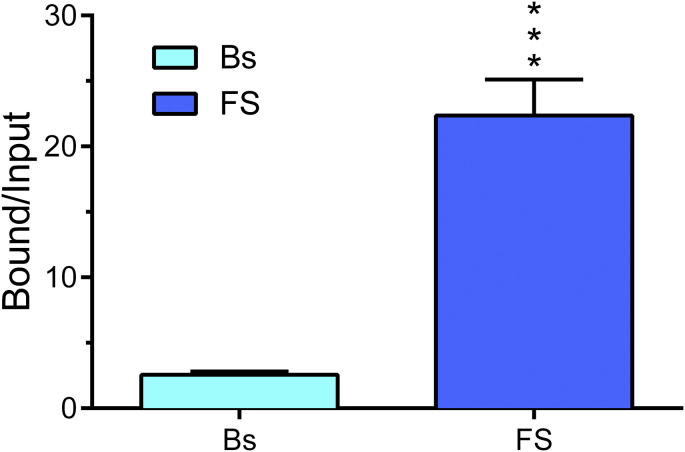
Classically, GRs and MRs act by binding as ligand-dependent transcription factor to gene promoter and other sites within the genome containing the consensus sequence of the so-called Glucocorticoid-Response Element (GRE). They can bind as homo-dimers as well as hetero-dimers (Trapp et al., 1994). Although the genomic action of GRs, and less so MRs, have been well investigated it is presently unclear whether such action and the consequences of such action in terms of specific gene output play a role in the behavioral responses discussed here. A study of Melly Oitzl and colleagues suggests that a genomic action of GRs may be important as well. A study using mice carrying a point-mutation that prevents homo-dimerization and hence DNA binding reported that these animals show impaired spatial memory formation in the Morris water maze with no changes in locomotion or anxiety-related behaviors (Oitzl et al., 2001). Thus, a role of genomic action of GR (and MR) and its consequences regarding gene expression needs to be further investigated. Approaches like chromatin-immuno-precipitation (ChIP) in combination with quantitative PCR have opened the possibility to study the binding of GRs and MRs to specific GRE sequences within gene promoters. Fig. 1 shows preliminary data of GR binding to a GRE within the promoter region of the Period 1 (Per1) gene using chromatin prepared from neocortex of rats killed at baseline or after forced swimming. Per1 is a GR-responsive period gene involved in circadian activities including the regulation of neuronal activity. Combination of ChIP with next-generation sequencing technologies allows studying GR binding across the entire genome. These relatively new epigenetic technologies in the field of neuroscience (Lester et al., 2011) should boost research output regarding the (epi)genomic action of GR and MR during the coming years.
Fig. 1.
Forced swimming results in significantly enhanced binding of GRs to a GRE sequence within the Per1 gene promoter. In an xChIP assay, cross-linked chromatin samples prepared from neocortex tissue of baseline (Bs) and forced swim (FS) rats were incubated with anti-GR antibody (Santa Cruz, CA). Bound and Input GRE-containing Per1 gene promoter DNA were quantified using qPCR. Data are expressed as the ratio of Bound/Input; mean ± SEM, n = 5. ∗, P < 0.001, Student's t-test.
It's becoming increasingly clear that glucocorticoids act on neuronal function through a great number of molecular mechanisms within different time domains. The fastest action is via membrane-bound receptors (Groeneweg et al., 2012), an issue which hasn't been addressed as their role in the behaviors mentioned here is unclear. The second fastest is the interaction of receptors with signaling mechanisms like the GR-MAPK interaction addressed here. The slowest one is the action of MRs and GRs (via GREs) at the genome. This molecular portfolio allows glucocorticoids to adjust neuron function via disparate mechanisms and different time domains, which underscores its importance for resilience.
6. Exercise: a boost for resilience
It is now well established that life style choices play a pivotal role in staying healthy and well, both physically and mentally. A life style option which has been obtaining great attention over the past several decades is physical activity. Initially, great benefits as a result of performing exercise regularly were seen with regard to cardiovascular health and controlling body weight. Presently, however, it has become clear that regular physical activity evokes vast changes in a plethora of body functions, many of which can be regarded as particularly beneficial for resilience. As the breadth of its effects on the body and mind is probably greater than any other life style option (e.g. meditation, yoga) we have chosen to review here the consequences of regular exercise with special emphasis regarding its benefits for stress resilience.
6.1. Exercise – the model
During the past 15 years evidence has been accumulating that an active life style is beneficial for resilience against stress. Often (in the media) it is thought that regular exercise is predominantly helpful for cardiovascular health and maintaining body weight in a healthy range. However, a variety of studies, exploring effects of exercise at the molecular, cellular, physiological and behavioral level, have shown that exercise has a deep impact on many body functions. When considering animal studies a distinction needs to be made between voluntary exercise and forced exercise. In the voluntary exercise paradigm, rodents like rats and mice run in a running wheel whenever they please to do so; they are not forced whatsoever. If provided with a running wheel they will run during the first half of the nighttime, i.e. the time when they are normally most active (Droste et al., 2003, Droste et al., 2007). A vast body of work indicates that this voluntary exercise has major beneficial effects and increases resilience to stress (Reul and Droste, 2005, Collins et al., 2012, van Praag et al., 1999). In contrast, the application of forced exercise, usually delivered by placing the animal for a period of time on a motorized treadmill during the daytime (when rats and mice normally sleep), is regarded as aversive by rodents and not beneficial in terms of increasing resilience (Stallknecht et al., 1990, Schmidt et al., 1992, Bedford et al., 1979). We will focus here on the voluntary exercise model. Several weeks of wheel running has indeed a major effect on body composition, but not really on body weight (Droste et al., 2003, Droste et al., 2007). Exercising rats and mice have substantially less abdominal fat and more muscle tissue.
6.2. Exercise – HPA axis changes
Long-term voluntary exercise has a major impact on physiological system like the HPA axis, the sympathetic nervous system and sleep regulation. Wheel running for several weeks evokes major changes in HPA axis regulation (Droste et al., 2003, Droste et al., 2007). These were associated with increased activity of the sympatho-adrenomedullary system, i.e. enhanced synthesis and release of adrenaline from the adrenal medulla, which is under sympathetic control (Droste et al., 2003, Droste et al., 2007). Exercising rats and mice show increases in adrenal weight (relative to the body weight; Reul and Droste, 2005, Droste et al., 2003, Droste et al., 2007). The adrenal medulla of the runners presented increased levels of tyrosine hydroxylase (TH; the rate-limiting enzyme in adrenaline synthesis) mRNA indicating a rise in the activity of sympatho-adrenomedullary system (Reul and Droste, 2005, Droste et al., 2003, Droste et al., 2007). These changes in adrenal size and adrenomedullary activity can be regarded as a direct consequence of long-term enhanced physical activity. Baseline early morning plasma ACTH levels were decreased in exercising mice suggesting a reduced hypothalamic-pituitary drive at this time of the day (Droste et al., 2003). Furthermore, evening plasma corticosterone values were higher in the running mice which may be an adaptive response to increased metabolic demand due to running during this time of the day/night cycle (Droste et al., 2003). In vivo microdialysis in exercising rats showed that free glucocorticoid hormone levels were increased at this time of the day as well (Droste et al., 2009b). There were distinct changes in the HPA axis responses to different stressful challenges. Exposure to a novel environment, which is regarded as a mild psychological stressor, resulted in a lower plasma glucocorticoid hormone response in exercising rats and mice than in sedentary animals (Droste et al., 2003, Droste et al., 2007). In contrast, subjecting rats and mice to forced swimming (this involves a substantial physical stress component) led to a significantly higher glucocorticoid response in the exercising animals (Droste et al., 2003, Droste et al., 2007). As plasma ACTH responses were not different to either stressor, it appears that mechanisms at the level of the adrenal gland are predominantly responsible for the distinct glucocorticoid responses to the novelty challenge and the forced swim stress. Due to its strong physical component forced swimming would evoke a strong release of adrenaline from the adrenal medulla, which is known to enhance the (ACTH-induced) secretion of corticosterone from the adrenal cortex (Bornstein et al., 1990, Bornstein et al., 2000, Engeland and Arnhold, 2005). In this regard, the enlarged adrenal cortex in exercising rats and mice would benefit a greater glucocorticoid response as well. To explain the diminished glucocorticoid response to novelty in the face of unchanged ACTH responses is not as straightforward. The presumably neural component responsible for suppressing the glucocorticoid response to novelty in the adrenal glands of exercising animals is still elusive.
In view of the enlarged adrenals in exercising animals the thought could arise whether these changes are adaptive or maladaptive as in chronic stress conditions enlarged adrenal glands have been observed as well. It is however unlikely that long-term voluntary exercise is comparable to a chronic stress condition. In exercising rats and mice we observed highly distinct glucocorticoid responses to novelty and forced swimming whilst ACTH responses were unchanged (Droste et al., 2003, Droste et al., 2007). In chronically stressed animals, in general, enhanced responses in ACTH and corticosterone to acute (heterotypic) stressors have been observed (Bhatnagar and Dallman, 1998). Furthermore, except for increased hippocampal GR mRNA levels, no changes were observed in brain MR and GR mRNA levels and paraventricular CRF, arginine-vasopressin (AVP) and oxytocin mRNA levels in long-term exercising rats (Droste et al., 2007). In chronic stress paradigms, usually MR and/or GR mRNA levels are decreased and CRF and AVP mRNA levels are increased. Thus, there are clear distinctions with regard to HPA axis changes between these models. Moreover, based on various observations on changes in cell biology (e.g. neurogenesis), physiology and behavior, exercise results in adaptive changes (Droste et al., 2003, Droste et al., 2007, Lancel et al., 2003, Binder et al., 2004a, van Praag et al., 1999) whereas the changes in chronic stress conditions are generally considered to be maladaptive (e.g. reduced neurogenesis, impaired structural plasticity, aberrant anxiety-related and social behavior) (McEwen, 2001, Wood et al., 2008).
In follow-up work, to obtain further insight into the significance of the altered glucocorticoid responses to stress in the exercising animals we conducted a microdialysis study in 4-weeks exercising and sedentary rats. As mentioned before, with this approach the levels of the free, biologically available fraction of glucocorticoid hormone is assessed. To our surprise, we observed no differences between the free corticosterone responses in the sedentary and exercised rats to either stressor (Droste et al., 2009b). There were also no differences in circulating early morning and evening baseline CBG levels between these animals. It may be speculated that exercising and sedentary animals show distinct responses in blood CBG levels after stress but this has not been investigated yet. Anyway, these ‘negative’ observations on free hormone responses generate some novel insights. First of all, measurement of total plasma glucocorticoid hormone only provides limited information about the real biologically active free concentration. Second, from a homeostatic perspective, it seems that, with regard to the free glucocorticoid hormone, the organism is keen to generate stressor-specific set response levels to stress. If like in the case of long-term exercise the enhanced sympatho-adrenomedullary drive results in enhanced total plasma corticosterone responses to physical challenges then apparently mechanisms are in place to adjust the available free hormone levels to match those in the sedentary animals. A similar mechanism is supposedly in place in case of mild psychological stressors. Identification of these mechanism(s) is important, as they are part of the nuts and bolts that constitute resilience. Consequently, disturbances in these adjusting mechanisms would result in hypo- or hyper-levels of glucocorticoid hormone, which could lead to development of various disorders.
We would like to note that in addition to exercise, gender is another example in which this mechanism of free glucocorticoid adjustment may be operational. It's known for many years that female rats and mice have substantially higher baseline and stress-induced total plasma glucocorticoid levels than their male counterparts. Using microdialysis, we found however that the free corticosterone levels at baseline and after stress were very similar between female and male rats (Droste et al., 2009a).
6.3. Exercise – sleep/EEG changes
In a sleep physiological study we studied various properties of the sleep/EEG pattern in exercising and sedentary mice including the duration of sleep episodes, sleep intensity, rapid eye movement (REM) sleep, non-REM sleep and wakefulness. These properties are indicators of sleep quality. For more information about our method of sleep recording, sleep analysis and spectrum analysis see Lancel et al. (1997).
We observed that long-term wheel running mice showed significantly less sleep episodes, however, these episodes were of longer duration indicating a better sleep consolidation (Lancel et al., 2003). Compared with sedentary controls the exercising mice also showed less REM sleep. A 15 min social conflict resulted in an increase in non-REM sleep, enhancement of low-frequency activity in the EEG within non-REM sleep (indicating increased sleep intensity) and less wakefulness in both control and exercising mice. In the control mice however an increased REM sleep concurrently with the rise in non-REM sleep was observed. In contrast, exercising animals showed a decrease in REM sleep. As the exercising animals showed higher plasma glucocorticoid responses after the stressor and glucocorticoids were known to inhibit REM sleep, it was thought at the time that the exercising mice showed a decrease in REM sleep after stress because of the higher glucocorticoid responses in these animals (Lancel et al., 2003). Presently, based on in vivo microdialysis studies (see above), we know that control and exercising animals do not differ regarding their free glucocorticoid hormone responses, so differential hormone responses cannot explain the distinct REM sleep responses in sedentary versus exercising mice. REM sleep is regulated by the activity of GABAergic neurons (Brooks and Peever, 2011). We have reported that exercising animals present changes in their GABAergic system (Hill et al., 2010), which could play a role in their altered REM sleep responses to stress. Further research is required to elucidate the role of this inhibitory neurotransmitter system in REM sleep regulation in exercising subjects. Nevertheless, our sleep data suggest that the beneficial effects of physical activity on resilience involve effects on sleep/EEG regulation. Through improvement of sleep consolidation and lengthening the duration of sleep episodes, regular physical exercise clearly increases sleep quality. Also in humans physical exercise has been shown to decrease overall REM sleep (Torsvall et al., 1984, Kupfer et al., 1985, Netzer et al., 2001). Studies on chronic stress in animals and major depressive illness in humans show that these conditions have deleterious effects on sleep quality and sleep/EEG. Chronic mild stress in rats shortens the duration of sleep episodes, thereby disrupting sleep maintenance, and raises the number of REM sleep episodes and overall REM sleep (Willner et al., 1992, Grønli et al., 2002). Disturbed sleep is one of the hallmarks of major depression. Depressed patients show a highly fragmented sleep, increased REM sleep and a shortened REM sleep latency (Kupfer, 1995). It is thought that clinically efficacious anti-depressant drugs reverse the sleep disturbances (Winokur et al., 2001). Clearly, in conditions like chronic stress and major depression resilience mechanisms are failing. Conversely, it seems that the effects of regular physical exercise on sleep/EEG strengthens resilience but more research is required in order to understand the underlying mechanisms and to gain better insight into the physiological significance of these effects.
6.4. Exercise – behavioral changes
Long-term voluntary exercise has vast effects on stress-related behavior in rats and mice indicating that exercise indeed strengthens resilience at the behavioral level. One of the earliest observations regarding the behavioral impact of exercise is the finding that wheel-running mice show improved spatial memory formation in the Morris water maze (van Praag et al., 1999). Notably, submission to this hippocampus-associated behavioral test is stressful for rats and mice as underlined by the significant rise in circulating plasma glucocorticoid hormone over the course of training (Carter S.D., Mifsud K.R. & Reul J.M.H.M., unpublished observations). Basically, the animal wishes to leave the water as quickly as possible and uses spatial cues (and memory formation thereof) to learn to find the platform speedily to this effect. Exercising mice learn faster than sedentary animals in this test (van Praag et al., 1999) suggesting that they are better in cognitively coping with the adverse situation. A similar conclusion could be drawn when exercising and sedentary rats were subjected to the forced swim test. Both groups of rats showed similar behaviors in the initial test. In the re-test 24 h later however the exercising rats showed significantly more immobility behavior and less struggling and swimming indicating an improved learned coping response in these animals (Collins et al., 2009).
Using various tests we reported that long-term exercising mice and rats show substantially less anxiety-related behavior (Binder et al., 2004a). Initially, when 4-weeks exercising mice were tested in an open field test the result was somewhat ambiguous. When the exercising mice were introduced to the open field they showed an increased delay before exploring the open field which could be interpreted as the result of an elevated anxiety state. However, the exercising animals compensated at a later stage of the test when they increasingly explored all areas of the open field. To obtain certainty about the anxiety state of the exercising mice we subjected them to the elevated plus-maze and the dark–light box, i.e. two established tests for anxiety-related behavior. In both tests, the exercising mice showed clear evidence for a reduced anxiety state as compared to the sedentary controls (Binder et al., 2004a). This reduced anxiety state after voluntary exercise has also been reported by other investigators (Duman et al., 2008). Thus, the initial delay in the open field test could not be explained by increased anxiety. We had observed that the exercising mice scanned the open field before embarking on its exploration. In view of these observations and findings of others that exercising animals have improved cognitive abilities, we hypothesized that the delay before exploration was the result of reduced impulsiveness. An initial delay was not only observed in the open field test but also in the so-called modified hole board test (Binder et al., 2004a). Nevertheless, the reduced impulsivity hypothesis, though intriguing, needs to be tested in appropriate behavioral tests.
Previously, we described that long-term exercising rats show reduced glucocorticoid hormone responses to a 30 min novelty (new clean cage) challenge (Droste et al., 2007). We postulated that this decreased neuroendocrine response in stress hormone secretion could be the result of reduced anxiety in these animals. Investigation of the control and exercising rats in the novel cage revealed a marked difference in the behavior of these animals under these psychologically stressful conditions (Droste et al., 2007, Collins et al., 2009). Typically, the sedentary rats explored the novel cage for the full 30 min. In contrast, the exercising animals showed over time significantly less exploration behavior (walking and rearing). A remarkable observation was that during the second half of the novelty exposure these rats showed a progressive increase in lying and resting/sleeping behavior (Droste et al., 2007, Collins et al., 2009). We concluded that exercising rats are substantially quicker in assessing a new environment regarding its potential dangers (and opportunities) and after this assessment has been made these animals return to their normal behavior for this time of the day (early morning) which is resting and sleeping. This rapid assessment capability in the physically active animals is most likely the result of enhanced cognitive abilities in combination with a reduced state of anxiety. These observations underscore the benefit of regular physical activity for boosting resilience.
6.5. Exercise – changes in pERK1/2–pMSK1/2 signaling and IEG induction – role of GABA?
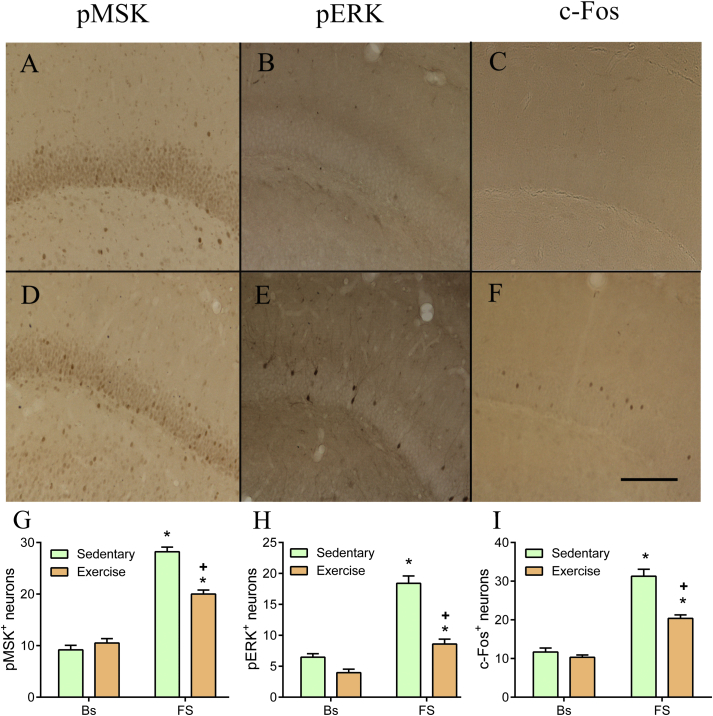
To obtain insight into the molecular mechanisms underlying the behavioral changes brought about by regular physical exercise we investigated the role of the signaling molecules pERK1/2 and pMSK1/2 and the IEG product c-Fos after forced swimming. As a detailed survey of pERK1/2 and pMSK1/2 had never been undertaken before, we assessed the immuno-reactivity of these molecules in many nuclei throughout the brain focusing on those brain regions known to be involved in the stress response. In control (sedentary) rats at baseline, the number of pERK1/2-positive (pERK+) neurons was very low in the neocortex, except for the moderate numbers found in the piriform cortex (Collins A. & Reul J.M.H.M, unpublished). At 15 min after the start of forced swimming (15 min, 25 C water) the number of pERK+ neurons had moderately to strongly increased in the cingulate, somatosensory, motor, perirhinal, prelimbic and infralimbic cortex but not in the piriform cortex. Moderate to strong increases were observed in the lateral septal nucleus, nucleus accumbens, locus coeruleus and dorsal raphe nucleus whereas no effects or small effects were observed in the magnocellular and parvocellular neurons of the hypothalamic PVN, central, medial and lateral nucleus of the amygdala, globus pallidus, caudate putamen, and median raphe nucleus. In the hippocampus, as shown before (Gutierrez-Mecinas et al., 2011), strong increases in pERK+ neurons were selectively found in the dorsal blade of the dentate gyrus (Fig. 2) whereas no or only small increments were found in the ventral blade of the dentate gyrus, CA1, CA2 and CA3 (Collins A. & Reul J.M.H.M, unpublished).
Fig. 2.
Effect of long-term voluntary exercise on forced swimming-induced MSK1/2 and ERK1/2 phosphorylation and c-Fos induction in rat dentate gyrus neurons. Male Sprague Dawley rats had 4 weeks access to a running wheel in their home cages after which they were either killed under baseline conditions (Bs) or at 15 min (pMSK, pERK) or at 60 min after start of forced swimming (FS; 15 min, 25 C water). Sacrifice and immunohistochemical procedures (including used antibodies) were exactly performed as described in Gutierrez-Mecinas et al. (2011). A–F: representative images of the dorsal blade of the dentate gyrus of baseline (A–C) and stressed (D–F) sedentary control rats. G–I: Quantitation of immuno-positive neurons in the dentate gyrus of sedentary and exercised rats killed at baseline or after forced swimming. Data are expressed as the average counts in a coronal 50 μm section (for details, see Gutierrez-Mecinas et al., 2011); mean ± SEM, n = 4 rats. ∗, P < 0.05, significantly different from the respective baseline group; +, P < 0.05, significantly different from the sedentary FS group, post-hoc Bonferroni test after two-way ANOVA. Scale bar is 100 µm.
In the neocortex of sedentary rats, the number of pMSK1/2-positive (pMSK+) neurons (presenting as nuclear staining) was low under baseline conditions except in the piriform cortex where numbers were already high under these conditions. Moderate increases in pMSK+ neurons were found at 15 min after the start of forced swimming in the cingulate, motor, perirhinal, prelimbic and the infralimbic cortex, but not in the somatosensory and piriform cortex (Collins A. & Reul J.M.H.M., unpublished). In addition, strong increases in pMSK+ neurons were observed in the lateral septal nucleus, nucleus accumbens, dorsal raphe nucleus and locus coeruleus but no effects were found in the central, medial and lateral nucleus of the amygdala, globus pallidus, caudate putamen and median raphe nucleus. At baseline, pMSK staining was considerable in both magnocellular and parvocellular neurons of the hypothalamic PVN but did not change after forced swimming. In all sub-regions of the hippocampus pMSK1/2 was very low to absent at baseline but after forced swimming a large increase was observed in the dorsal blade of the dentate gyrus (as reported before (Gutierrez-Mecinas et al., 2011); Fig. 2) and only small increases were found in the CA1 and CA2. In the other sub-regions, including the ventral blade of the dentate gyrus and CA3, no changes were observed.
The forced swimming-induced changes in c-Fos expression (at 60 min after the start of forced swimming) in the brain of sedentary rats were similar to the pattern we reported many years ago (Bilang-Bleuel et al., 2002). In control rats, moderate to strong effects of forced swimming were found throughout the neocortex, lateral septal nucleus, hypothalamic PVN, nucleus accumbens, caudate putamen, and locus coeruleus. In the hippocampus, a strong increase was observed in the dorsal blade of the dentate gyrus 60 min after the start of forced swim stress (Fig. 2) but in the other regions including the dentate's ventral blade (Gutierrez-Mecinas et al., 2011), CA1, CA2 and CA3 hardly any or very small effects were observed (Collins A and Reul J.M.H.M., unpublished).
We investigated the effects of long-term voluntary exercise on baseline and forced swimming-induced changes in pMSK+, pERK+ and c-Fos+ neurons in the brain. To our surprise we only found significant effects of regular physical activity on pERK1/2, pMSK1/2 and c-Fos responses in the dentate gyrus (Fig. 2). Exercise had no effect on baseline levels but it substantially attenuated the effect of forced swimming on the responses in pERK1/2, pMSK1/2 and c-Fos in dentate gyrus granule neurons (Fig. 2). The effect of forced swimming and the attenuating effect of exercise were selectively found in the dorsal blade of the dentate gyrus (Collins A. and Reul J.M.H.M., unpublished). In a previous study (Collins et al., 2009), we had investigated the effect of forced swimming on H3S10p-K14ac and c-Fos in dentate gyrus granule neurons of exercising rats killed at 2 h after forced swimming. We found that at that time point the stressor resulted in a significantly higher response in histone H3 phospho-acetylation and c-Fos induction in the runners than in the non-runners (Collins et al., 2009). It appears that an initial suppression of responses was over-compensated at a later point in time, the underlying mechanism of which is presently unclear. Thus, regular physical activity alters the behavioral immobility response and, as shown here, targets signaling and IEG responses in the dentate gyrus that are implemented in the consolidation of this behavioral response.
The attenuating effects of exercise on the initial forced swimming-induced molecular responses in the dentate gyrus may correspond with the reduced state of anxiety in exercising animals. The change in emotionality in these animals may be the result of adjustments in the GABAergic system. We had published that, besides distinct changes in the expression of GABA-A receptor subunits (e.g. the extra-synaptic receptor associated delta and alpha-5 subunits), regular physical activity led to increased gene transcription of the GABA-synthesizing enzyme GAD67 (Hill et al., 2010). Moreover, our recent preliminary data indicate that GABA synthesis is increased in the dentate gyrus/CA3 of exercising rats (Kersanté et al., unpublished observations). This is an important observation as we have previously reported that GABAergic neurotransmission is a critical regulator of stress-evoked (pERK1/2- and pMSK1/2-targeted) epigenetic and IEG transcriptional responses in the dentate gyrus (Papadopoulos et al., 2008). We found that a single injection of a non-sedative dose of the anxiolytic benzodiazepine, Lorazepam (a GABA-A receptor allosteric modulator) blocked the novelty stress-induced rise in H3S10p-K14ac- and c-Fos-positive granule neurons in the dentate gyrus. Moreover, administration of the partial inverse agonist FG7142 resulted in strongly enhanced novelty-induced increases in H3S10p-K14ac- and c-Fos-positive neurons in the dentate gyrus (Papadopoulos et al., 2008). FG7142 has been shown to be an anxiogenic drug in rats and humans by lowering GABA-A receptor function (Dorow et al., 1983, Kalueff and Nutt, 1996, Evans and Lowry, 2007). Additional information on the role of anxiety state and GABAergic neurotransmission on epigenetic, gene transcriptional and behavioral responses can be found elsewhere (Reul, 2014). Collectively, it seems that the beneficial effects of regular physical exercise on anxiety state and behavioral responses involve the enhancement of GABAergic inhibitory control. Thus, in addition to glucocorticoids, GABA may be an important mediator of the positive effects of exercise on resilience.
6.6. Exercise – controversial findings on mood in humans
Studies on the effects of regular exercise (and physical activity in general) on mood and affect in humans have been conducted over the past 20 years. The outcome picture has been rather mixed. For instance, some studies have been published showing improvements in measures of anxiety and depression (Steptoe et al., 1989, Byrne and Byrne, 1993, Salmon, 2001) whereas an investigation looking into the effects of ‘facilitated physical activity’ in addition to usual care (antidepressant treatment) reported no significant effects (Chalder et al., 2012). Cooney and colleagues recently conducted a meta-analysis involving 2326 participants on the effectiveness of exercise in the treatment of depression in adults compared with no treatment (or placebo treatment) or a comparator intervention (pharmacological or psychological treatment) (Cooney et al., 2013). Overall, exercise was more effective than no or placebo treatment in reducing depressive symptoms and equally effective as pharmacological and psychological treatment (Cooney et al., 2013). The extent of efficacy of exercise was reduced if only methodologically robust trials were considered. A few months ago these authors wrote in a JAMA Synopsis review: “Exercise is associated with a greater reduction in depression symptoms compared with no treatment, placebo, or active control interventions, such as relaxation or meditation. However, analysis of high-quality studies alone suggests only small benefits.” (Cooney et al., 2014). Presently, several points can be made. First of all, more methodologically robust studies should be conducted. By nature, exercise studies in humans are difficult to design. Questions like which exercise to apply (e.g. aerobic, anaerobic, endurance or just facilitated physical activity?), how often and how long (days, weeks, months?) and which patients to include/exclude need to be answered. Different modes of applied exercise will invariably result in variations in outcome. Blinding of treatments is inherently difficult in exercise studies. Human studies suffer from variability by nature as humans differ greatly in terms of physical and physiological properties and responses. Furthermore, major depressive and anxiety disorders are very heterogeneous psychiatric disorders, a situation which may greatly contribute to the variation in treatment outcome. Voluntary exercise studies on mice and rats produce much less variability as all animals will be of the same sex and similar weight/age and will receive the same exercise, i.e. usually a running wheel. There may be differences among animals in running wheel performance (in km/day) but, at least in our hands using male Sprague Dawley rats and male C57/Bl6 mice, this has made no difference in terms of the extent of HPA axis and behavioural changes (Reul JMHM and Droste SK, unpublished observations).
If the verdict ultimately is that the efficacy of exercise is not greater than that of pharmacological or psychological treatment, this would not be entirely disappointing. It needs to be considered that exercise has no adverse side effects which unfortunately cannot be said of pharmacological treatments. Furthermore, given that exercise has positive effects on the body and mind besides its effects on mood and affective state, it will contribute to the general health and wellbeing of the individual.
With regard to human studies on exercise mostly the effects of exercise and physical activity on patients suffering from depression and/or anxiety have been investigated. In view of consideration how to effectively enhance stress resilience in individuals, future studies should be directed toward the question whether an active physical life style provides increased protection against the impact of traumatic experiences in terms of PTSD and other mental disorders.
7. Fkbp5 in stress resilience and vulnerability
The FK506 binding protein 51 or Fkbp5 was first identified as a novel steroid hormone receptor binding protein over 20 years ago (Sanchez, 1990), and research has revealed that it plays a prominent role in stress-related diseases (Zannas and Binder, 2014, Binder, 2009). Fkbp5 is a co-chaperone and interacts with the GR through the heat shock protein HSP90 (Jaaskelainen et al., 2011). When Fkbp5 is bound to the GR complex cortisol binds with lower affinity and nuclear translocation of the receptor is reduced; thus Fkbp5 acts as a negative regulator of GR function (Jaaskelainen et al., 2011). In fact, GR activation rapidly induces Fkbp5 mRNA and protein expression thus creating a short, negative feedback loop that regulates GR function (Binder, 2009, Jaaskelainen et al., 2011). Furthermore, Fkbp5 is also a co-chaperone of other steroid receptors including the progesterone and androgen receptors (Stechschulte and Sanchez, 2011); however, in contrast to the effects on the GR, Fkbp5 increases the sensitivity of the androgen receptor (Stechschulte and Sanchez, 2011). The human Fkbp5 gene locus spans approximately 155 kbp on the short arm of chromosome 6 and the gene contains 13 exons (Jaaskelainen et al., 2011) with GREs found throughout the gene; however, functional GREs have only been shown to be present upstream of the promoter region, and in introns 2, 5 and 7 (Zannas and Binder, 2014, Jaaskelainen et al., 2011, Paakinaho et al., 2010). It is believed that these GRE enhancers come into direct contact with the transcription start site and RNA polymerase II via the formation of three-dimensional (3D) chromatin loops (Klengel and Binder, 2013a, Jaaskelainen et al., 2011), consequently promoting a glucocorticoid-induced increase in Fkbp5 gene transcription.
Genetic variations in the Fkbp5 region are associated with regulation of the HPA axis, resulting in an altered responsiveness to stress, which seems to predispose an individual to psychiatric disorders. A number of studies have shown association of Fkbp5 polymorphisms with an increased susceptibility to major depression (Lavebratt et al., 2010, Lekman et al., 2008, Zimmermann et al., 2011, Zobel et al., 2010), bipolar disorder (Willour et al., 2009) and posttraumatic stress disorder (PTSD) (Appel et al., 2011, Binder et al., 2008, Mehta et al., 2011, Sarapas et al., 2011, Xie et al., 2010) as well as an increased suicide risk (Brent et al., 2010, Roy et al., 2012, Supriyanto et al., 2011), especially in interaction with exposure to early trauma.
Binder et al. (2004b) have described polymorphisms in the promoter region, intron 2 and the 3′ un-translated region of human Fkbp5 – these three single nucleotide polymorphisms (SNPs) rs4713916, rs1360780, and rs3800373, were associated with increased recurrence of depressive episodes and rapid antidepressant response (Lekman et al., 2008, Binder et al., 2004b). In particular, rs1360780 T allele which is located close to a functional GRE in intron 2 is associated with greater induction of Fkbp5 mRNA with GR activation, leading to compromised negative feedback of the stress hormone system (Klengel and Binder, 2013a, Binder et al., 2004b). It is thought that direct contact of the intron 2 GRE with the transcription start site is enhanced in T allele carriers (Klengel and Binder, 2013a). In addition, studies have shown that healthy subjects who are carriers of the rs1360780 T allele show protracted cortisol responses to psychosocial stress (Ising et al., 2008, Luijk et al., 2010), suggesting that the GR is showing some resistance in these individuals. Moreover, Binder et al. (2008) reported that in an African–American sample, four SNPs (rs3800373, rs9296158, rs1360780, and rs9470080) interacted with childhood trauma in predicting symptoms of posttraumatic stress disorder (PTSD), a disorder associated with both a raised risk of attempting suicide and HPA axis dysregulation (Binder et al., 2008, Wilcox et al., 2009). Therefore, it appears that Fkbp5 can moderate the influence of childhood trauma on the stress-responsive HPA axis.
Changes in the methylation status of cytosine nucleotides within the genomic DNA are an established epigenetic mechanism, which regulates gene expression and plays a pivotal role in neural plasticity and environmental adaptation (Telese et al., 2013). Furthermore, changes in DNA methylation in response to traumatic experiences and stress are now thought to play an important role in stress-related psychiatric disorders (Klengel et al., 2014). A recent study has shown that allele specific changes in DNA methylation induced by early trauma bring about the interaction observed between child abuse and Fkbp5 in the development of stress-related psychiatric disorders (Klengel and Binder, 2013a). This study found that rs1360780 T allele carriers who were exposed to child abuse, show de-methylation of CpGs in the functional GRE in intron 7 of the Fkbp5 gene. This de-methylation of CpGs in intron 7, leads to an enhanced induction of Fkbp5 transcription by GR agonists and is associated with GR resistance. Interestingly, in carriers of the rs1360780C allele, trauma-induced de-methylation of intron 7 GRE is absent. Furthermore, de-methylation in this region of FKBP5 was only dependent on exposure to child abuse but not dependent on exposure to adult trauma. Thus, de-methylation of the GRE region in intron 7 results in an enhanced stressor-evoked induction of Fkbp5 and impaired GR-mediated negative feedback of the HPA axis (Klengel and Binder, 2013a). Together, these findings support the idea that exposure of children to abuse who carry risk alleles in Fkbp5, which can cause enduring epigenetic changes in Fkbp5 gene expression, are predisposed to stress-associated disorders such as PTSD. Thus, GR and Fkbp5 molecules form an intricate reciprocal control loop that is of critical importance for stress resilience and health. Polymorphisms have also been found in the GR. Although it is still early days, associations between SNPs within GR and phenotype have been described for metabolism, body composition, the immune and cardiovascular systems, and psychiatric diseases (Koper et al., 2014, in press). However, as the frequency of most SNPs is rather low, it has been suggested that the influence of a single SNP on health and disease is limited (Koper et al., 2014, in press).
8. Early life stress, epigenetic changes and long-term consequences for resilience
Resilience in adulthood is impaired during episodes of chronic depression, PTSD and other mental disorders. Clinical studies into the origin of chronic depression found childhood adversity, in the form of parental neglect, physical and/or sexual abuse, to be one of the main factors in predicting episodes of chronic depression in adulthood based on a sample of 404 women (Brown and Moran, 1994, Brown et al., 1994). Other researchers have shown that a history of childhood adversity is predictive for other mood, anxiety, behavioral and substance disorders including bipolar disorder, PTSD, ADHD and drug/alcohol misuse respectively, although it should be noted that many studies are limited in some way either by the retrospective analysis of abuse or influencing factors not taken into consideration (Kessler et al., 2010). Despite the strong correlation between early life stress and mental illness, according to the Connar-Davidson Resilience Scale (CDRISC) the presence of resilience characteristics such as hardiness, tenacity and adaptability can mitigate the negative outcome of early childhood stress on some of these disorders (Wingo et al., 2010, Wingo et al., 2014).
Research into the physiological effects of childhood adversity on stress-coping systems, namely the HPA axis identified complex changes in both the ovine CRF-activated HPA response and the exogenous ACTH-evoked response in circulating glucocorticoid levels (Heim et al., 2001). Thus, whereas the CRF-induced increase in plasma ACTH levels was enhanced in women with a history of childhood abuse but without comorbid major depressive disorder (MDD), a blunted ACTH response was found in women with MDD irrespective of the presence of childhood abuse. Interestingly, only in abused women without comorbid MDD, baseline cortisol levels and the cortisol response to synthetic ACTH were decreased (Heim et al., 2001). In a further study, Heim et al. (2000) investigated the HPA axis responses to psychosocial stress, which, rather than the pharmacological challenges, involves higher cognitive and emotional processing (Heim et al., 2000). Women with a history of childhood abuse (physical or sexual) had significantly higher levels of ACTH released following psychosocial stress compared with non-abused women regardless of mental state. The observation that the abused women presenting with MDD have an exaggerated ACTH response to psychosocial stress but not to pharmacological stimulation could indicate that additional cognitive and emotional elements involved in this stress are interacting with pathways or emotions established from a history of childhood abuse. Subsequent enhanced responses in circulating cortisol levels and heart rate to psychosocial stress were only observed in abused women presenting with MDD in adulthood but not in abused women without MDD, despite exaggerated ACTH responses in both groups. Taken together, these findings indicate that childhood abuse precipitates pituitary sensitization with subsequent counter-regulatory adrenocortical adaptations occurring only in abused women without MDD, which may be regarded as a potential form of resilience (Heim et al., 2008). Exposure to further life stressors may lead to the HPA axis profile seen in the group of abused women with comorbid MDD and thus it seems that resilience is compromised in these women.
Long-term changes in HPA axis function due to experiences encountered during childhood have been widely attributed to changes in the epigenome. Early studies of Michael Meaney's group investigating the effects of maternal behavior on the offspring's HPA axis function in adulthood provided the first evidence for an epigenetic link between early-life experiences and life-long changes in HPA axis function (Weaver et al., 2004). Rat pups reared by high care-giving mothers exhibited a sustained DNA de-methylation in the promoter region of the GR gene within the hippocampus shortly after birth. This DNA de-methylation was associated with enhanced acetylation of lysine 9 within histone H3 and increased Egr-1 binding, promoting gene transcription. In contrast, rats reared from low care-giving mothers had significant re-methylation of this region after birth leading to aberrant HPA axis function and anxiety-like behavior in adulthood (Weaver et al., 2004). In later studies it was found that maternal care also resulted in de-methylation of the region responsible for maternal behavior in female offspring, namely the estrogen receptor alpha 1b of the medial preoptic area (Champagne et al., 2006). These epigenetic changes in the estrogen receptor determined which class of care-giver female pups would become based on their experience as pups. Hence, female offspring of low care-giving dams would become low care-giving dams and propagate the cycle of epigenetic changes based on maternal care (Champagne et al., 2006).
Other components of the HPA axis have been investigated for epigenetic changes as a result of early life stress (ELS) including the proopiomelanocortin (POMC) gene which is responsible for producing the pro-hormone for ACTH production (Patchev et al., 2014). Daily separation of mice pups from their mother (a form of ELS) disrupted basal corticosterone levels in offspring compared with controls (non-ELS) and detailed epigenetic analysis of the POMC gene in the ELS mice identified a sustained hypo-methylation in the distal promoter region in ELS mice compared with non-ELS controls. This hypo-methylation was functionally linked to an increase in POMC mRNA expression possibly as a result of decreased binding of protein methyl-CpG-binding protein 2 (Mecp2) and DNA-methyltransferase 1 (DNMT1), which are involved in transcriptional repression. These epigenetic changes in the POMC gene, as a result of ELS, were still present in aged mice tested at 1 year (Patchev et al., 2014).
McGowan et al. (2009) translated the animal studies described above regarding the GR gene into the human situation of child-abuse related suicide and found similar epigenetic changes as those identified within the hippocampal GR promoter of low-care giving rats to those present in the human hippocampal GR gene promoter (McGowan et al., 2009). Male suicide victims abused as children had increased methylation of the hippocampal GR promoter region and an associated reduction in GR gene transcription compared with hippocampal samples from non-abused suicide victims or age-matched non-suicide non-abused controls. Later studies examining changes in the blood of children and adolescents with or without a history of childhood abuse have revealed that: 1. Changes in DNA methylation patterns occur shortly after the adverse experience (van der Knaap et al., 2014, Romens et al., 2014); 2. Increases in DNA methylation within the GR promoter region as a result of childhood adversity is not exclusive to the hippocampus and can be detected in DNA extracted from whole blood (van der Knaap et al., 2014, Romens et al., 2014); and 3. DNA methylation levels in the promoter region of the GR gene are positively correlated with the number of stressful life events (such as parental divorce, hospitalization, parental illness etc.) a child or young adult experiences in a cumulative manner (van der Knaap et al., 2014). Additional genome-wide screening studies have been performed on both human blood (Bick et al., 2012, Suderman et al., 2014) and brain tissue (Labonte et al., 2012) to identify the sheer number of genes differentially methylated when categorized based on experience of childhood abuse.
The relevance of long lasting epigenetic changes as a result of early life experiences could be explained by the emerging match/mismatch hypothesis of psychiatric disease (Nederhof, 2012). Studies on human development (reviewed in Belsky and Pluess (2009)) discussed the possibility that apparent ‘negative’ behavioral and or molecular changes occurring as a result of adverse environmental experience during development may, in fact, increase resilience when dealing with a matched environment of high stress in later life. These ideas forming the basis of match/mismatch hypothesis of psychiatric disease suggest that individuals are better suited when adapting to an environment which matches their early life experience (Nederhof and Schmidt, 2012). Mice experiencing mismatched environmental conditions between early life and adulthood had lower thymus weight and reduced basal corticosterone levels compared with mice experiencing both positive and negatively matched environments (Santarelli et al., 2014). When facing an adverse challenge, in the form of the forced swim test, mice that had experienced early life stress were quicker to adapt to the stressful experience compared with mice that had experienced a beneficial early care regime (Santarelli et al., 2014). Maternal separation in early life also had an enhancing effect on freezing behavior when rats were exposed to fear conditioning following a chronic stress paradigm in adulthood compared with non-maternally separated rats indicating the adverse experience of maternal separation had increased the adaptive response of the rats to stressful situations in adulthood and supporting the match/mismatch hypothesis (Zalosnik et al., 2014). Taken together these studies may indicate that whilst early life stress causes long term changes in the HPA axis and stress response these may be designed to increase resilience of that individual to stress in later life but clearly more research is needed to verify the validity of the match/mismatch hypothesis.
9. Perspectives
Resilience is of crucial importance for maintaining health throughout life. It may be regarded as an important factor in the mitigation of allostatic load, i.e. the slipping of homeostatic mechanisms due to genetic vulnerabilities in combination with the adversities of life (McEwen, 2001, McEwen, 2012a). Research over the past seven decades has made it undeniably clear that glucocorticoid hormones play a pivotal role in processes underlying adaptation and resilience. Not surprisingly, glucocorticoid function is highly regulated to safeguard the organism from hypo- as well as hyper-function of this steroid hormone. As illustrated in this article, the regulation of glucocorticoid function is taking place at multiple levels: 1. Through the tight control of biologically available hormone for binding to MRs and GRs during baseline and stress conditions, and other physiological conditions like exercise, resulting in differential MR and GR occupancies. These hormone concentrations are kept in check within the HPA axis through intricate ultradian and circadian, feed-forward and feed-back mechanisms, and a plethora of HPA axis-afferent systems such as the sympathetic nervous system and the central aminergic systems; 2. Through the regulation of the concentration of MRs and GRs in various tissues during baseline and stress conditions and over the life span; 3. Through the fine-tuning of MR and GR activities by co-chaperone molecules like Fkbp5 and many other steroid receptor co-regulators; 4. Through interaction of MRs and GRs with activated or induced signaling molecules whose availability depends on the state of cellular activity. These interactions determine epigenetic modifications, gene promoter accessibility, transcription factor activities (including those of MRs and GRs themselves!) and thus gene transcription (Reul, 2014). This list doesn't claim to be exhaustive and new mechanisms are still being discovered, and no doubt, with future discoveries possible.
With all the checks and balances in place it appears that the entire system or network controlling glucocorticoid function and resilience is rather robust. In principle this may be the case, yet more than 10% of our population is suffering from stress-related major depressive disorder and anxiety-related disorders. It appears that the system can fail if put under high strain, such as major (chronic) emotional stress, in combination with genetic vulnerability (SNPs, point mutations) in key molecules. Genetic vulnerabilities in particular have a substantial, often life-long impact, if physical or sexual abuse occurs during early childhood with a significantly higher risk of developing major depressive disorder or anxiety disorders in later life.
These novel insights into the effects of stress and glucocorticoids on the brain, particularly in relation to the role of epigenetic control of gene expression and its consequences for neuronal function and behavior, will help to develop new treatment strategies for patients suffering from a stress-related mental disorder. In this respect, the combined application of epigenetic techniques and whole genome screening technologies in the neuroscience of stress resilience will accelerate the accumulation of vital knowledge. In addition to the development of novel pharmacological treatments, attention should be given to the neurobiology underlying the beneficial effects of life style choices such as exercise, mindfulness and meditation.
Acknowledgments
Our work described in this paper has been supported by BBSRC grants BB/F006802/1, BB/G02507X/1 and BB/K007408/1, the Wellcome Trust grant 092947/Z/10/Z, and MRC capacity building PhD studentships to AC and SDC.
References
- Appel K., Schwahn C., Mahler J., Schulz A., Spitzer C., Fenske K., Stender J., Barnow S., John U., Teumer A., Biffar R., Nauck M., Volzke H., Freyberger H.J., Grabe H.J. Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology. 2011;36:1982–1991. doi: 10.1038/npp.2011.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arriza J.L., Weinberger C., Cerelli G., Glaser T.M., Handelin B.L., Housman D.E., Evans R.M. Cloning of human mineralocorticoid receptor complementary DNA: structural and functional kinship with the glucocorticoid receptor. Science. 1987;237:268–275. doi: 10.1126/science.3037703. [DOI] [PubMed] [Google Scholar]
- Bedford T.G., Tipton C.M., Wilson N.C., Oppliger R.A., Gisolfi C.V. Maximal oxygen consumption of rats and its changes with various experimental procedures. J. Appl. Physiol. 1979;47:1278–1283. doi: 10.1152/jappl.1979.47.6.1278. [DOI] [PubMed] [Google Scholar]
- Belsky J., Pluess M. Beyond diathesis stress: differential susceptibility to environmental influences. Psychol. Bull. 2009;135:885–908. doi: 10.1037/a0017376. [DOI] [PubMed] [Google Scholar]
- Beylin A.V., Shors T.J. Glucocorticoids are necessary for enhancing the acquisition of associative memories after acute stressful experience. Horm. Behav. 2003;43:124–131. doi: 10.1016/s0018-506x(02)00025-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatnagar S., Dallman M. Neuroanatomical basis for facilitation of hypothalamic–pituitary–adrenal responses to a novel stressor after chronic stress. Neuroscience. 1998;84:1025–1039. doi: 10.1016/s0306-4522(97)00577-0. [DOI] [PubMed] [Google Scholar]
- Bick J., Naumova O., Hunter S., Barbot B., Lee M., Luthar S.S., Raefski A., Grigorenko E.L. Childhood adversity and DNA methylation of genes involved in the hypothalamus–pituitary–adrenal axis and immune system: whole-genome and candidate-gene associations. Dev. Psychopathol. 2012;24:1417–1425. doi: 10.1017/S0954579412000806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilang-Bleuel A., Rech J., Holsboer F., Reul J.M.H.M. Forced swimming evokes a biphasic response in CREB phosphorylation in extrahypothalamic limbic and neocortical brain structures. Eur. J. Neurosci. 2002;15:1048–1060. doi: 10.1046/j.1460-9568.2002.01934.x. [DOI] [PubMed] [Google Scholar]
- Bilang-Bleuel A., Ulbricht S., Chandramohan Y., De Carli S., Droste S.K., Reul J.M.H.M. Psychological stress increases histone H3 phosphorylation in adult dentate gyrus granule neurons: involvement in a glucocorticoid receptor-dependent behavioural response. Eur. J. Neurosci. 2005;22:1691–1700. doi: 10.1111/j.1460-9568.2005.04358.x. [DOI] [PubMed] [Google Scholar]
- Binder E.B. The role of FKBP5, a co-chaperone of the glucocorticoid receptor in the pathogenesis and therapy of affective and anxiety disorders. Psychoneuroendocrinology. 2009;34(Suppl. 1):S186–S195. doi: 10.1016/j.psyneuen.2009.05.021. [DOI] [PubMed] [Google Scholar]
- Binder E., Droste S.K., Ohl F., Reul J.M.H.M. Regular voluntary exercise reduces anxiety-related behaviour and impulsiveness in mice. Behav. Brain Res. 2004;155:197–206. doi: 10.1016/j.bbr.2004.04.017. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Salyakina D., Lichtner P., Wochnik G.M., Ising M., Putz B., Papiol S., Seaman S., Lucae S., Kohli M.A., Nickel T., Kunzel H.E., Fuchs B., Majer M., Pfennig A., Kern N., Brunner J., Modell S., Baghai T., Deiml T., Zill P., Bondy B., Rupprecht R., Messer T., Kohnlein O., Dabitz H., Bruckl T., Muller N., Pfister H., Lieb R., Mueller J.C., Lohmussaar E., Strom T.M., Bettecken T., Meitinger T., Uhr M., Rein T., Holsboer F., Muller-Myhsok B. Polymorphisms in FKBP5 are associated with increased recurrence of depressive episodes and rapid response to antidepressant treatment. Nat. Genet. 2004;36:1319–1325. doi: 10.1038/ng1479. [DOI] [PubMed] [Google Scholar]
- Binder E.B., Bradley R.G., Liu W., Epstein M.P., Deveau T.C., Mercer K.B., Tang Y., Gillespie C.F., Heim C.M., Nemeroff C.B., Schwartz A.C., Cubells J.F., Ressler K.J. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. JAMA. 2008;299:1291–1305. doi: 10.1001/jama.299.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornstein S.R., Ehrhart-Bornstein M., Scherbaum W.A., Pfeiffer E.F., Holst J.J. Effects of splanchnic nerve stimulation on the adrenal cortex may be mediated by chromaffin cells in a paracrine manner. Endocrinology. 1990;127:900–906. doi: 10.1210/endo-127-2-900. [DOI] [PubMed] [Google Scholar]
- Bornstein S.R., Tian H., Haidan A., Bottner A., Hiroi N., Eisenhofer G., Mccann S.M., Chrousos G.P., Roffler-Tarlov S. Deletion of tyrosine hydroxylase gene reveals functional interdependence of adrenocortical and chromaffin cell system in vivo. Proc. Natl. Acad. Sci. U. S. A. 2000;97:14742–14747. doi: 10.1073/pnas.97.26.14742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brent D., Melhem N., Ferrell R., Emslie G., Wagner K.D., Ryan N., Vitiello B., Birmaher B., Mayes T., Zelazny J., Onorato M., Devlin B., Clarke G., DeBar L., Keller M. Association of FKBP5 polymorphisms with suicidal events in the treatment of resistant depression in adolescents (TORDIA) study. Am. J. Psychiatry. 2010;167:190–197. doi: 10.1176/appi.ajp.2009.09040576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P.L., Peever J.H. Impaired GABA and glycine transmission triggers cardinal features of rapid eye movement sleep behavior disorder in mice. J. Neurosci. 2011;31:7111–7121. doi: 10.1523/JNEUROSCI.0347-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown G.W., Moran P. Clinical and psychosocial origins of chronic depressive episodes. I: a community survey. Br. J. Psychiatry. 1994;165:447–456. doi: 10.1192/bjp.165.4.447. [DOI] [PubMed] [Google Scholar]
- Brown G.W., Harris T.O., Hepworth C., Robinson R. Clinical and psychosocial origins of chronic depressive episodes. II. A patient enquiry. Br. J. Psychiatry. 1994;165:457–465. doi: 10.1192/bjp.165.4.457. [DOI] [PubMed] [Google Scholar]
- Byrne A., Byrne D.G. The effect of exercise on depression, anxiety and other mood states: a review. J. Psychosom. Res. 1993;37:565–574. doi: 10.1016/0022-3999(93)90050-p. [DOI] [PubMed] [Google Scholar]
- Carroll J.C., Iba M., Bangasser D.A., Valentino R.J., James M.J., Brunden K.R., Lee V.M., Trojanowski J.Q. Chronic stress exacerbates tau pathology, neurodegeneration, and cognitive performance through a corticotropin-releasing factor receptor-dependent mechanism in a transgenic mouse model of tauopathy. J. Neurosci. 2011;31:14436–14449. doi: 10.1523/JNEUROSCI.3836-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalder M., Wiles N.J., Campbell J., Hollinghurst S.P., Haase A.M., Taylor A.H., Fox K.R., Costelloe C., Searle A., Baxter H., Winder R., Wright C., Turner K.M., Calnan M., Lawlor D.A., Peters T.J., Sharp D.J., Montgomery A.A., Lewis G. Facilitated physical activity as a treatment for depressed adults: randomised controlled trial. BMJ. 2012;344:e2758. doi: 10.1136/bmj.e2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne F.A., Weaver I.C., Diorio J., Dymov S., Szyf M., Meaney M.J. Maternal care associated with methylation of the estrogen receptor-alpha1b promoter and estrogen receptor-alpha expression in the medial preoptic area of female offspring. Endocrinology. 2006;147:2909–2915. doi: 10.1210/en.2005-1119. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y., Droste S.K., Reul J.M.H.M. Novelty stress induces phospho-acetylation of histone H3 in rat dentate gyrus granule neurons through coincident signalling via the N-methyl-d-aspartate receptor and the glucocorticoid receptor: relevance for c-fos induction. J. Neurochem. 2007;101:815–828. doi: 10.1111/j.1471-4159.2006.04396.x. [DOI] [PubMed] [Google Scholar]
- Chandramohan Y., Droste S.K., Arthur J.S., Reul J.M.H.M. The forced swimming-induced behavioural immobility response involves histone H3 phospho-acetylation and c-Fos induction in dentate gyrus granule neurons via activation of the N-methyl-d-aspartate/extracellular signal-regulated kinase/mitogen- and stress-activated kinase signalling pathway. Eur. J. Neurosci. 2008;27:2701–2713. doi: 10.1111/j.1460-9568.2008.06230.x. [DOI] [PubMed] [Google Scholar]
- Cheung P., Tanner K.G., Cheung W.L., Sassone-Corsi P., Denu J.M., Allis C.D. Synergistic coupling of histone H3 phosphorylation and acetylation in response to epidermal growth factor stimulation. Mol. Cell. 2000;5:905–915. doi: 10.1016/s1097-2765(00)80256-7. [DOI] [PubMed] [Google Scholar]
- Cheung P., Allis C.D., Sassone-Corsi P. Signaling to chromatin through histone modifications. Cell. 2000;103:263–271. doi: 10.1016/s0092-8674(00)00118-5. [DOI] [PubMed] [Google Scholar]
- Chwang W.B., Arthur J.S., Schumacher A., Sweatt J.D. The nuclear kinase mitogen- and stress-activated protein kinase 1 regulates hippocampal chromatin remodeling in memory formation. J. Neurosci. 2007;27:12732–12742. doi: 10.1523/JNEUROSCI.2522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Hill L.E., Chandramohan Y., Whitcomb D., Droste S.K., Reul J.M.H.M. Exercise improves cognitive responses to psychological stress through enhancement of epigenetic mechanisms and gene expression in the dentate gyrus. PLoS ONE. 2009;4:e4330. doi: 10.1371/journal.pone.0004330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins A., Gutierrez-Mecinas M., Trollope A.F., Reul J.M.H.M. Epigenetics of stress. In: Parrizas M., Gasa R., Kaliman P., editors. Epigenetics of Lifestyle. Bentham Science Publishers; Dubai: 2012. pp. 70–89. [Google Scholar]
- Cooney G.M., Dwan K., Greig C.A., Lawlor D.A., Rimer J., Waugh F.R., McMurdo M., Mead G.E. Exercise for depression. Cochrane Database Syst. Rev. 2013;9:CD004366. doi: 10.1002/14651858.CD004366.pub6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooney G.M., Dwan K., Mead G. Exercise for depression. JAMA. 2014;311:2432–2433. doi: 10.1001/jama.2014.4930. [DOI] [PubMed] [Google Scholar]
- Cordero M.I., Sandi C. A role for brain glucocorticoid receptors in contextual fear conditioning: dependence upon training intensity. Brain Res. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Reul J.M.H.M. Feedback action and tonic influence of corticosteroids on brain function: a concept arising from the heterogeneity of brain receptor systems. Psychoneuroendocrinology. 1987;12:83–105. doi: 10.1016/0306-4530(87)90040-0. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Reul J.M.H.M., Van den Bosch F.R., Tonnaer J.A.D.M., Saito H. Ginsenoside RG1 and corticosteroid receptors in rat brain. Endocrinol. Jpn. 1987;34:213–220. doi: 10.1507/endocrj1954.34.213. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., De Kock S., Schild V., Veldhuis H.D. Antiglucocorticoid RU 38486 attenuates retention of a behaviour and disinhibits the hypothalamic–pituitary adrenal axis at different brain sites. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Vreugdenhil E., Oitzl M.S., Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr. Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- De Kloet E.R., Joëls M., Holsboer F. Stress and the brain: from adaptation to disease. Nat. Rev. Neurosci. 2005;6:463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- De Pablo J.M., Parra A., Segovia S., Guillamón A. Learned immobility explains the behavior of rats in the forced swim test. Physiol. Behav. 1989;46:229–237. doi: 10.1016/0031-9384(89)90261-8. [DOI] [PubMed] [Google Scholar]
- Dorow R., Horowski R., Paschelke G., Amin M. Severe anxiety induced by FG 7142, a beta-carboline ligand for benzodiazepine receptors. Lancet. 1983;2:98–99. doi: 10.1016/s0140-6736(83)90076-4. [DOI] [PubMed] [Google Scholar]
- Droste S.K., de Groote L., Lightman S.L., Reul J.M.H.M., Linthorst A.C. The ultradian and circadian rhythms of free corticosterone in the brain are not affected by gender: an in vivo microdialysis study in Wistar rats. J. Neuroendocrinol. 2009;21:132–140. doi: 10.1111/j.1365-2826.2008.01811.x. [DOI] [PubMed] [Google Scholar]
- Droste S.K., Gesing A., Ulbricht S., Muller M.B., Linthorst A.C.E., Reul J.M.H.M. Effects of long-term voluntary exercise on the mouse hypothalamic–pituitary–adrenocortical axis. Endocrinology. 2003;144:3012–3023. doi: 10.1210/en.2003-0097. [DOI] [PubMed] [Google Scholar]
- Droste S.K., Chandramohan Y., Reul J.M.H.M. Voluntary exercise impacts on the rat hypothalamic–pituitary–adrenal axis mainly at the adrenal level. Neuroendocrinology. 2007;86:26–37. doi: 10.1159/000104770. [DOI] [PubMed] [Google Scholar]
- Droste S.K., de G.L., Atkinson H.C., Lightman S.L., Reul J.M.H.M., Linthorst A.C.E. Corticosterone levels in the brain show a distinct ultradian rhythm but a delayed response to forced swim stress. Endocrinology. 2008;149:3244–3253. doi: 10.1210/en.2008-0103. [DOI] [PubMed] [Google Scholar]
- Droste S.K., Collins A., Lightman S.L., Linthorst A.C.E., Reul J.M.H.M. Distinct, time-dependent effects of voluntary exercise on circadian and ultradian rhythms and stress responses of free corticosterone in the rat hippocampus. Endocrinology. 2009;150:4170–4179. doi: 10.1210/en.2009-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman C.H., Schlesinger L., Russell D.S., Duman R.S. Voluntary exercise produces antidepressant and anxiolytic behavioral effects in mice. Brain Res. 2008;1199:148–158. doi: 10.1016/j.brainres.2007.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeland W.C., Arnhold M.M. Neural circuitry in the regulation of adrenal corticosterone rhythmicity. Endocrine. 2005;28:325–332. doi: 10.1385/ENDO:28:3:325. [DOI] [PubMed] [Google Scholar]
- Evans A.K., Lowry C.A. Pharmacology of the beta-carboline FG-7142, a partial inverse agonist at the benzodiazepine allosteric site of the GABA A receptor: neurochemical, neurophysiological, and behavioral effects. CNS Drug Rev. 2007;13:475–501. doi: 10.1111/j.1527-3458.2007.00025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuxe K., Wikström A.-C., Okret S., Agnati L.F., Härfstrand A., Yu Z.-Y., Granholm L., Zoli M., Vale W., Gustafsson J.-A. Mapping of glucocorticoid receptor immunoreactive neurons in the rat tel- and diencephalon using a monoclonal antibody against rat liver glucocorticoid receptor. Endocrinology. 1985;177:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- Fuxe K., Wilström A.-C., Okret S., Agnati L.F., Härfstrand A., Yu Z.-Y., Granholm L., Zoli M., Vale W., Gustafsson J.A. Mapping of glucocorticoid receptor mRNA in the male rat brain by in situ hybridization. Endocrinology. 1985;117:1803–1812. doi: 10.1210/endo-117-5-1803. [DOI] [PubMed] [Google Scholar]
- Gesing A., Bilang-Bleuel A., Droste S.K., Linthorst A.C.E., Holsboer F., Reul J.M.H.M. Psychological stress increases hippocampal mineralocorticoid receptor levels: involvement of corticotropin-releasing hormone. J. Neurosci. 2001;21:4822–4829. doi: 10.1523/JNEUROSCI.21-13-04822.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groeneweg F.L., Karst H., De Kloet E.R., Joëls M. Mineralocorticoid and glucocorticoid receptors at the neuronal membrane, regulators of nongenomic corticosteroid signalling. Mol. Cell. Endocrinol. 2012;350:299–309. doi: 10.1016/j.mce.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Grønli J., Murison R., Bjorvatn B., Portas C., Ursin R. Sleep/wake effect after chronic mild stress in rats. J. Sleep Res. 2002;11(Suppl. 1):87. [Google Scholar]
- Gutierrez-Mecinas M., Trollope A.F., Collins A., Morfett H., Hesketh S.A., Kersante F., Reul J.M.H.M. Long-lasting behavioral responses to stress involve a direct interaction of glucocorticoid receptors with ERK1/2–MSK1–Elk-1 signaling. Proc. Natl. Acad. Sci. U. S. A. 2011;108:13806–13811. doi: 10.1073/pnas.1104383108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond G.L. Molecular properties of corticosteroid binding globulin and the sex-steroid binding proteins. Endocr. Rev. 1990;11:65–79. doi: 10.1210/edrv-11-1-65. [DOI] [PubMed] [Google Scholar]
- Hammond G.L., Smith C.L., Underhill D.A. Molecular studies of corticosteroid binding globulin structure, biosynthesis and function. J. Steroid Biochem. Mol. Biol. 1991;40:755–762. doi: 10.1016/0960-0760(91)90300-t. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Heit S., Graham Y.P., Wilcox M., Bonsall R., Miller A.H., Nemeroff C.B. Pituitary–adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA. 2000;284:592–597. doi: 10.1001/jama.284.5.592. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Bonsall R., Miller A.H., Nemeroff C.B. Altered pituitary–adrenal axis responses to provocative challenge tests in adult survivors of childhood abuse. Am. J. Psychiatry. 2001;158:575–581. doi: 10.1176/appi.ajp.158.4.575. [DOI] [PubMed] [Google Scholar]
- Heim C., Newport D.J., Mletzko T., Miller A.H., Nemeroff C.B. The link between childhood trauma and depression: insights from HPA axis studies in humans. Psychoneuroendocrinology. 2008;33:693–710. doi: 10.1016/j.psyneuen.2008.03.008. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Cullinan W.E. Neurocircuitry of stress: central control of the hypothalamo–pituitary–adrenocortical axis. Trends Neurosci. 1997;20:78–84. doi: 10.1016/s0166-2236(96)10069-2. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Patel P.D., Akil H., Watson S.J. Localization and regulation of glucocorticoid and mineralocorticoid receptor messenger RNAs in the hippocampal formation of the rat. Mol. Endocrinol. 1989;3:1886–1894. doi: 10.1210/mend-3-11-1886. [DOI] [PubMed] [Google Scholar]
- Herman J.P., Schäfer M.K.H., Young E.A., Thompson R., Douglass J., Akil H., Watson S.J. Evidence for hippocampal regulation of neuroendocrine neurons of the hypothalamo–pituitary–adrenocortical axis. J. Neurosci. 1989;9:3072–3082. doi: 10.1523/JNEUROSCI.09-09-03072.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman J.P., Figueiredo H., Mueller N.K., Ulrich-Lai Y., Ostrander M.M., Choi D.C., Cullinan W.E. Central mechanisms of stress integration: hierarchical circuitry controlling hypothalamo–pituitary–adrenocortical responsiveness. Front. Neuroendocrinol. 2003;24:151–180. doi: 10.1016/j.yfrne.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Hill L.E., Droste S.K., Nutt D.J., Linthorst A.C.E., Reul J.M.H.M. Voluntary exercise alters GABAA receptor subunit and glutamic acid decarboxylase-67 gene expression in the rat forebrain. J. Psychopharmacol. 2010;24:745–756. doi: 10.1177/0269881108096983. [DOI] [PubMed] [Google Scholar]
- Hollenberg S.M., Weinberger C., Ong E.S., Cerelli G., Oro A., Lebo R., Thompson E.B., Rosenfeld M.G., Evans R.M. Primary structure and expression of a functional human glucocorticoid receptor cDNA. Nature. 1985;318:635–641. doi: 10.1038/318635a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ising M., Depping A.M., Siebertz A., Lucae S., Unschuld P.G., Kloiber S., Horstmann S., Uhr M., Muller-Myhsok B., Holsboer F. Polymorphisms in the FKBP5 gene region modulate recovery from psychosocial stress in healthy controls. Eur. J. Neurosci. 2008;28:389–398. doi: 10.1111/j.1460-9568.2008.06332.x. [DOI] [PubMed] [Google Scholar]
- Jaaskelainen T., Makkonen H., Palvimo J.J. Steroid up-regulation of FKBP51 and its role in hormone signaling. Curr. Opin. Pharmacol. 2011;11:326–331. doi: 10.1016/j.coph.2011.04.006. [DOI] [PubMed] [Google Scholar]
- Jefferys D., Copolov D., Irby D., Funder J. Behavioural effect of adrenalectomy: reversal by glucocorticoids or (D-ALA2,MET5)enkephalinamide. Eur. J. Pharmacol. 1983;92:99–103. doi: 10.1016/0014-2999(83)90113-9. [DOI] [PubMed] [Google Scholar]
- Joëls M., De Kloet E.R. Control of neuronal excitability by corticosteroid hormones. Trends Neurosci. 1992;15:25–30. doi: 10.1016/0166-2236(92)90345-9. [DOI] [PubMed] [Google Scholar]
- Joëls M., Krugers H.J., Lucassen P.J., Karst H. Corticosteroid effects on cellular physiology of limbic cells. Brain Res. 2009;1293:91–100. doi: 10.1016/j.brainres.2009.03.036. [DOI] [PubMed] [Google Scholar]
- Kalueff A., Nutt D.J. Role of GABA in memory and anxiety. Depress. Anxiety. 1996;4:100–110. doi: 10.1002/(SICI)1520-6394(1996)4:3<100::AID-DA2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Kessler R.C., McLaughlin K.A., Green J.G., Gruber M.J., Sampson N.A., Zaslavsky A.M., Aguilar-Gaxiola S., Alhamzawi A.O., Alonso J., Angermeyer M., Benjet C., Bromet E., Chatterji S., de G.G., Demyttenaere K., Fayyad J., Florescu S., Gal G., Gureje O., Haro J.M., Hu C.Y., Karam E.G., Kawakami N., Lee S., Lepine J.P., Ormel J., Posada-Villa J., Sagar R., Tsang A., Ustun T.B., Vassilev S., Viana M.C., Williams D.R. Childhood adversities and adult psychopathology in the WHO World Mental Health Surveys. Br. J. Psychiatry. 2010;197:378–385. doi: 10.1192/bjp.bp.110.080499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss J.Z., Van Eekelen J.A.M., Reul J.M.H.M., Westphal H.M., De Kloet E.R. Glucocorticoid receptor in magnocellular neurosecretory cells. Endocrinology. 1988;122:444–449. doi: 10.1210/endo-122-2-444. [DOI] [PubMed] [Google Scholar]
- Klengel T., Binder E.B. Allele-specific epigenetic modification: a molecular mechanism for gene–environment interactions in stress-related psychiatric disorders? Epigenomics. 2013;5:109–112. doi: 10.2217/epi.13.11. [DOI] [PubMed] [Google Scholar]
- Klengel T., Binder E.B. Gene–environment interactions in major depressive disorder. Can. J. Psychiatry. 2013;58:76–83. doi: 10.1177/070674371305800203. [DOI] [PubMed] [Google Scholar]
- Klengel T., Pape J., Binder E.B., Mehta D. The role of DNA methylation in stress-related psychiatric disorders. Neuropharmacology. 2014;80:115–132. doi: 10.1016/j.neuropharm.2014.01.013. [DOI] [PubMed] [Google Scholar]
- Koper J.W., van Rossum E.F., van den Akker E.L. Glucocorticoid receptor polymorphisms and haplotypes and their expression in health and disease. Steroids. 2014 doi: 10.1016/j.steroids.2014.07.015. (in press) [DOI] [PubMed] [Google Scholar]
- Korte S.M. Corticosteroids in relation to fear, anxiety and psychopathology. Neurosci. Biobehav. Rev. 2001;25:117–142. doi: 10.1016/s0149-7634(01)00002-1. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J. Sleep research in depressive illness: clinical implications – a tasting menu. Biol. Psychiatry. 1995;38:391–403. doi: 10.1016/0006-3223(94)00295-E. [DOI] [PubMed] [Google Scholar]
- Kupfer D.J., Sewitch D.E., Epstein L.H., Bulik C., McGowen C.R., Robertson R.J. Exercise and subsequent sleep in male runners: failure to support the slow wave sleep-mood-exercise hypothesis. Neuropsychobiology. 1985;14:5–12. doi: 10.1159/000118193. [DOI] [PubMed] [Google Scholar]
- Labeur M.S., Arzt E., Wiegers G.J., Holsboer F., Reul J.M.H.M. Long-term intracerebroventricular corticotropin-releasing hormone administration induces distinct changes in rat splenocyte activation and cytokine expression. Endocrinology. 1995;136:2678–2688. doi: 10.1210/endo.136.6.7750492. [DOI] [PubMed] [Google Scholar]
- Labonte B., Suderman M., Maussion G., Navaro L., Yerko V., Mahar I., Bureau A., Mechawar N., Szyf M., Meaney M.J., Turecki G. Genome-wide epigenetic regulation by early-life trauma. Arch. Gen. Psychiatry. 2012;69:722–731. doi: 10.1001/archgenpsychiatry.2011.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancel M., Faulhaber J., Schiffelholz T., Mathias S., Deisz R.A. Muscimol and midazolam do not potentiate each other's effects on sleep EEG in the rat. J. Neurophysiol. 1997;77:1624–1629. doi: 10.1152/jn.1997.77.3.1624. [DOI] [PubMed] [Google Scholar]
- Lancel M., Droste S.K., Sommer S., Reul J.M.H.M. Influence of regular voluntary exercise on spontaneous and social stress-affected sleep in mice. Eur. J. Neurosci. 2003;17:2171–2179. doi: 10.1046/j.1460-9568.2003.02658.x. [DOI] [PubMed] [Google Scholar]
- Lavebratt C., Aberg E., Sjoholm L.K., Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J. Affect. Disord. 2010;125:249–255. doi: 10.1016/j.jad.2010.02.113. [DOI] [PubMed] [Google Scholar]
- Lekman M., Laje G., Charney D., Rush A.J., Wilson A.F., Sorant A.J., Lipsky R., Wisniewski S.R., Manji H., McMahon F.J., Paddock S. The FKBP5-gene in depression and treatment response – an association study in the sequenced treatment alternatives to relieve depression (STAR∗D) Cohort. Biol. Psychiatry. 2008;63:1103–1110. doi: 10.1016/j.biopsych.2007.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester B.M., Tronick E., Nestler E., Abel T., Kosofsky B., Kuzawa C.W., Marsit C.J., Maze I., Meaney M.J., Monteggia L.M., Reul J.M.H.M., Skuse D.H., Sweatt J.D., Wood M.A. Behavioral epigenetics. Ann. N. Y. Acad. Sci. 2011;1226:14–33. doi: 10.1111/j.1749-6632.2011.06037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst A.C., Reul J.M. Stress and the brain: solving the puzzle using microdialysis. Pharmacol. Biochem. Behav. 2008;90:163–173. doi: 10.1016/j.pbb.2007.09.019. [DOI] [PubMed] [Google Scholar]
- Linthorst A.C.E., Flachskamm C., Holsboer F., Reul J.M.H.M. Local administration of recombinant human interleukin-1 beta in the rat hippocampus increases serotonergic neurotransmission, hypothalamic–pituitary–adrenocortical axis activity, and body temperature. Endocrinology. 1994;135:520–532. doi: 10.1210/endo.135.2.7518383. [DOI] [PubMed] [Google Scholar]
- Linthorst A.C.E., Flachskamm C., Müller-Preuss P., Holsboer F., Reul J.M.H.M. Effect of bacterial endotoxin and interleukin-1 beta on hippocampal serotonergic neurotransmission, behavioral activity, and free corticosterone levels: an in vivo microdialysis study. J. Neurosci. 1995;15:2920–2934. doi: 10.1523/JNEUROSCI.15-04-02920.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst A.C.E., Flachskamm C., Hopkins S.J., Hoadley M.E., Labeur M.S., Holsboer F., Reul J.M.H.M. Long-term intracerebroventricular infusion of corticotropin-releasing hormone alters neuroendocrine, neurochemical, autonomic, behavioral, and cytokine responses to a systemic inflammatory challenge. J. Neurosci. 1997;17:4448–4460. doi: 10.1523/JNEUROSCI.17-11-04448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linthorst A.C.E., Peñalva R.G., Flachskamm C., Holsboer F., Reul J.M.H.M. Forced swim stress activates rat hippocampal serotonergic neurotransmission involving a corticotropin-releasing hormone receptor-dependent mechanism. Eur. J. Neurosci. 2002;16:2441–2452. doi: 10.1046/j.1460-9568.2002.02400.x. [DOI] [PubMed] [Google Scholar]
- Linthorst A.C.E., Flachskamm C., Reul J.M.H.M. Water temperature determines neurochemical and behavioural responses to forced swim stress: an in vivo microdialysis and biotelemetry study in rats. Stress. 2008;11:88–100. doi: 10.1080/10253890701533231. [DOI] [PubMed] [Google Scholar]
- Luijk M.P., Velders F.P., Tharner A., van Ijzendoorn M.H., Bakermans-Kranenburg M.J., Jaddoe V.W., Hofman A., Verhulst F.C., Tiemeier H. FKBP5 and resistant attachment predict cortisol reactivity in infants: gene–environment interaction. Psychoneuroendocrinology. 2010;35:1454–1461. doi: 10.1016/j.psyneuen.2010.04.012. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Plasticity of the hippocampus: adaptation to chronic stress and allostatic load. Ann. N. Y. Acad. Sci. 2001;933:265–277. doi: 10.1111/j.1749-6632.2001.tb05830.x. [DOI] [PubMed] [Google Scholar]
- McEwen B.S. Brain on stress: how the social environment gets under the skin. Proc. Natl. Acad. Sci. U. S. A. 2012;109(Suppl. 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen B.S. The ever-changing brain: cellular and molecular mechanisms for the effects of stressful experiences. Dev. Neurobiol. 2012;72:878–890. doi: 10.1002/dneu.20968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan P.O., Sasaki A., D'Alessio A.C., Dymov S., Labonte B., Szyf M., Turecki G., Meaney M.J. Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat. Neurosci. 2009;12:342–348. doi: 10.1038/nn.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta D., Gonik M., Klengel T., Rex-Haffner M., Menke A., Rubel J., Mercer K.B., Putz B., Bradley B., Holsboer F., Ressler K.J., Muller-Myhsok B., Binder E.B. Using polymorphisms in FKBP5 to define biologically distinct subtypes of posttraumatic stress disorder: evidence from endocrine and gene expression studies. Arch. Gen. Psychiatry. 2011;68:901–910. doi: 10.1001/archgenpsychiatry.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mifsud K.R., Gutierrez-Mecinas M., Trollope A.F., Collins A., Saunderson E.A., Reul J.M.H.M. Epigenetic mechanisms in stress and adaptation. Brain Behav. Immun. 2011;25:1305–1315. doi: 10.1016/j.bbi.2011.06.005. [DOI] [PubMed] [Google Scholar]
- Mitchell J.B., Meaney M.J. Effects of corticosterone on response consolidation and retrieval in the forced swim test. Behav. Neurosci. 1991;105:798–803. doi: 10.1037//0735-7044.105.6.798. [DOI] [PubMed] [Google Scholar]
- Muller M.B., Zimmermann S., Sillaber I., Hagemeyer T.P., Deussing J.M., Timpl P., Kormann M.S., Droste S.K., Kuhn R., Reul J.M.H.M., Holsboer F., Wurst W. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat. Neurosci. 2003;6:1100–1107. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- Munck A., Guyre P.M., Holbrook N.J. Physiological functions of glucocorticoids in stress and their relation to pharmacological actions. Endocr. Rev. 1984;5:25–44. doi: 10.1210/edrv-5-1-25. [DOI] [PubMed] [Google Scholar]
- Nederhof E. The mismatch hypothesis of psychiatric disease. Physiol. Behav. 2012;106:689–690. doi: 10.1016/j.physbeh.2012.02.014. [DOI] [PubMed] [Google Scholar]
- Nederhof E., Schmidt M.V. Mismatch or cumulative stress: toward an integrated hypothesis of programming effects. Physiol. Behav. 2012;106:691–700. doi: 10.1016/j.physbeh.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Widerlöv E., Bissette G., Walléus H., Karlsson I., Eklund K., Kilts C.D., Loosen P.T., Vale W. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Nemeroff C.B., Owens M.J., Bissette G., Andorn A.C., Stanley M. Reduced corticotropin-releasing factor binding sites in the frontal cortex of suicide victims. Arch. Gen. Psychiatry. 1988;45:577–579. doi: 10.1001/archpsyc.1988.01800300075009. [DOI] [PubMed] [Google Scholar]
- Netzer N.C., Kristo D., Steinle H., Lehmann M., Strohl K.P. REM sleep and catecholamine excretion: a study in elite athletes. Eur. J. Appl. Physiol. 2001;84:521–526. doi: 10.1007/s004210100383. [DOI] [PubMed] [Google Scholar]
- Nowak S.J., Corces V.G. Phosphorylation of histone H3 correlates with transcriptionally active loci. Genes Dev. 2000;14:3003–3013. doi: 10.1101/gad.848800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oitzl M.S., De Kloet E.R. Selective corticosteroid antagonists modulate specific aspects of spatial orientation learning. Behav. Neurosci. 1992;106:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- Oitzl M.S., Reichardt H.M., Joels M., De Kloet E.R. Point mutation in the mouse glucocorticoid receptor preventing DNA binding impairs spatial memory. Proc. Natl. Acad. Sci. U. S. A. 2001;98:12790–12795. doi: 10.1073/pnas.231313998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paakinaho V., Makkonen H., Jaaskelainen T., Palvimo J.J. Glucocorticoid receptor activates poised FKBP51 locus through long-distance interactions. Mol. Endocrinol. 2010;24:511–525. doi: 10.1210/me.2009-0443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papadopoulos A., Chandramohan Y., Collins A., Droste S.K., Nutt D.J., Reul J.M.H.M. GABAergic control of stress-responsive epigenetic and gene expression mechanisms in the dentate gyrus. Eur. Neuropsychopharmacol. 2008;18:S211–S212. doi: 10.1016/j.euroneuro.2010.05.005. [DOI] [PubMed] [Google Scholar]
- Patchev A.V., Rodrigues A.J., Sousa N., Spengler D., Almeida O.F. The future is now: early life events preset adult behaviour. Acta Physiol. (Oxf.) 2014;210:46–57. doi: 10.1111/apha.12140. [DOI] [PubMed] [Google Scholar]
- Pavlides C., Watanabe Y., McEwen B.S. Effects of glucocorticoids on hippocampal long-term potentiation. Hippocampus. 1993;3:183–192. doi: 10.1002/hipo.450030210. [DOI] [PubMed] [Google Scholar]
- Petersen H.H., Andreassen T.K., Breiderhoff T., Brasen J.H., Schulz H., Gross V., Grone H.J., Nykjaer A., Willnow T.E. Hyporesponsiveness to glucocorticoids in mice genetically deficient for the corticosteroid binding globulin. Mol. Cell. Biol. 2006;26:7236–7245. doi: 10.1128/MCB.00400-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Droste S.K., Gutierrez-Mecinas M., Collins A., Kersante F., Reul J.M.H.M., Linthorst A.C.E. A rapid release of corticosteroid-binding globulin from the liver restrains the glucocorticoid hormone response to acute stress. Endocrinology. 2011;152:3738–3748. doi: 10.1210/en.2011-1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian X., Droste S.K., Lightman S.L., Reul J.M.H.M., Linthorst A.C.E. Circadian and ultradian rhythms of free glucocorticoid hormone are highly synchronized between the blood, the subcutaneous tissue, and the brain. Endocrinology. 2012;153:4346–4353. doi: 10.1210/en.2012-1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M.H.M. Making memories of stressful events: a journey along epigenetic, gene transcription, and signaling pathways. Front. Psychiatry. 2014;5:5. doi: 10.3389/fpsyt.2014.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reul J.M.H.M., Chandramohan Y. Epigenetic mechanisms in stress-related memory formation. Psychoneuroendocrinology. 2007;32(Suppl. 1):S21–S25. doi: 10.1016/j.psyneuen.2007.03.016. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., De Kloet E.R. Two receptor systems for corticosterone in rat brain: microdistribution and differential occupation. Endocrinology. 1985;117:2505–2512. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., De Kloet E.R. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J. Steroid Biochem. 1986;24:269–272. doi: 10.1016/0022-4731(86)90063-4. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr. Opin. Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Van den Bosch F.R., De Kloet E.R. Relative occupation of type-I and type-II corticosteroid receptors in rat brain following stress and dexamethasone treatment: functional implications. J. Endocrinol. 1987;115:459–467. doi: 10.1677/joe.0.1150459. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Tonnaer J.A.D.M., De Kloet E.R. Neurotrophic ACTH analogue promotes plasticity of type I corticosteroid receptor in brain of senescent male rats. Neurobiol. Aging. 1988;9:253–260. doi: 10.1016/s0197-4580(88)80062-9. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., De Kloet E.R., Van Sluijs F.J., Rijnberk A., Rothuizen J. Binding characteristics of mineralocorticoid and glucocorticoid receptors in dog brain and pituitary. Endocrinology. 1990;127:907–915. doi: 10.1210/endo-127-2-907. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Rothuizen J., De Kloet E.R. Age-related changes in the dog hypothalamic–pituitary–adrenocortical system: neuroendocrine activity and corticosteroid receptors. J. Steroid Biochem. 1991;40:63–69. doi: 10.1016/0960-0760(91)90168-5. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Stec I., Söder M., Holsboer F. Chronic treatment of rats with the antidepressant amitriptyline attenuates the activity of the hypothalamic–pituitary–adrenocortical system. Endocrinology. 1993;133:312–320. doi: 10.1210/endo.133.1.8391426. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Labeur M.S., Grigoriadis D.E., De Souza E.B., Holsboer F. Hypothalamic–pituitary–adrenocortical axis changes in the rat after long-term treatment with the reversible monoamine oxidase-A inhibitor moclobemide. Neuroendocrinology. 1994;60:509–519. doi: 10.1159/000126788. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Gesing A., Droste S.K., Stec I.S.M., Weber A., Bachmann C.G., Bilang-Bleuel A., Holsboer F., Linthorst A.C.E. The brain mineralocorticoid receptor: greedy for ligand, mysterious in function. Eur. J. Pharmacol. 2000;405:235–249. doi: 10.1016/s0014-2999(00)00677-4. [DOI] [PubMed] [Google Scholar]
- Reul J.M.H.M., Droste S.K. The hypothalamic–pituitary–adrenal axis as a dynamically organized system: lessons from exercising mice. In: Steckler T., Kalin N.H., Reul J.M.H.M., editors. Handbook of Stress and the Brain – Part 1: the Neurobiology of Stress. Elsevier; Amsterdam: 2005. pp. 95–112. [Google Scholar]
- Reul J.M.H.M., Hesketh S.A., Collins A., Gutierrez-Mecinas M. Epigenetic mechanisms in the dentate gyrus act as a molecular switch in hippocampus-associated memory function. Epigenetics. 2009;4:434–439. doi: 10.4161/epi.4.7.9806. [DOI] [PubMed] [Google Scholar]
- Richard E.M., Helbling J.C., Tridon C., Desmedt A., Minni A.M., Cador M., Pourtau L., Konsman J.P., Mormede P., Moisan M.P. Plasma transcortin influences endocrine and behavioral stress responses in mice. Endocrinology. 2010;151:649–659. doi: 10.1210/en.2009-0862. [DOI] [PubMed] [Google Scholar]
- Rolls E.T., Kesner R.P. A computational theory of hippocampal function, and empirical tests of the theory. Prog. Neurobiol. 2006;79:1–48. doi: 10.1016/j.pneurobio.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Romens S.E., McDonald J., Svaren J., Pollak S.D. Associations between early life stress and gene methylation in children. Child Dev. 2014 doi: 10.1111/cdev.12270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothuizen J., Reul J.M.H.M., Van Sluijs F.J., Mol J.A., Rijnberk A., De Kloet E.R. Increased neuroendocrine reactivity and decreased brain mineralocorticoid receptor-binding capacity in aged dogs. Endocrinology. 1993;132:161–168. doi: 10.1210/endo.132.1.8380372. [DOI] [PubMed] [Google Scholar]
- Roy A., Hodgkinson C.A., Deluca V., Goldman D., Enoch M.A. Two HPA axis genes, CRHBP and FKBP5, interact with childhood trauma to increase the risk for suicidal behavior. J. Psychiatr. Res. 2012;46:72–79. doi: 10.1016/j.jpsychires.2011.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin. Psychol. Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Sanchez E.R. Hsp56-A novel heat shock protein associated with untransformed steroid receptor complexes. J. Biol. Chem. 1990;265:22067–22070. [PubMed] [Google Scholar]
- Sandi C., Loscertales M., Guaza C. Experience-dependent facilitating effect of corticosterone on spatial memory formation in the water maze. Eur. J. Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- Santarelli S., Lesuis S.L., Wang X.D., Wagner K.V., Hartmann J., Labermaier C., Scharf S.H., Muller M.B., Holsboer F., Schmidt M.V. Evidence supporting the match/mismatch hypothesis of psychiatric disorders. Eur. Neuropsychopharmacol. 2014;24:907–918. doi: 10.1016/j.euroneuro.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Sarapas C., Cai G., Bierer L.M., Golier J.A., Galea S., Ising M., Rein T., Schmeidler J., Muller-Myhsok B., Uhr M., Holsboer F., Buxbaum J.D., Yehuda R. Genetic markers for PTSD risk and resilience among survivors of the world trade center attacks. Dis. Mark. 2011;30:101–110. doi: 10.3233/DMA-2011-0764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K.N., Gosselin L.E., Stanley W.C. Endurance exercise training causes adrenal medullary hypetrophy in young and old Fischer 344 rats. Horm. Metab. Res. 1992;24:511–515. doi: 10.1055/s-2007-1003377. [DOI] [PubMed] [Google Scholar]
- Seckl J.R., Fink G. Use of in situ hybridization to investigate the regulation of hippocampal corticosteroid receptors by monoamines. J. Steroid Biochem. Mol. Biol. 1991;40:685–688. doi: 10.1016/0960-0760(91)90291-c. [DOI] [PubMed] [Google Scholar]
- Stallknecht B., Kjær M., Ploug T., Maroun L., Ohkuwa T., Vinten J., Mikines K.J., Galbo H. Diminished epinephrine response to hypoglycemia despite enlarged adrenal medulla in trained rats. Am. J. Physiol. 1990;259:R998–R1003. doi: 10.1152/ajpregu.1990.259.5.R998. [DOI] [PubMed] [Google Scholar]
- Stechschulte L.A., Sanchez E.R. FKBP51 – a selective modulator of glucocorticoid and androgen sensitivity. Curr. Opin. Pharmacol. 2011;11:332–337. doi: 10.1016/j.coph.2011.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A., Edwards S., Moses J., Mathews A. The effects of exercise training on mood and perceived coping ability in anxious adults from the general population. J. Psychosom. Res. 1989;33:537–547. doi: 10.1016/0022-3999(89)90061-5. [DOI] [PubMed] [Google Scholar]
- Suderman M., Borghol N., Pappas J.J., Pinto Pereira S.M., Pembrey M., Hertzman C., Power C., Szyf M. Childhood abuse is associated with methylation of multiple loci in adult DNA. BMC Med. Genomics. 2014;7:13. doi: 10.1186/1755-8794-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supriyanto I., Sasada T., Fukutake M., Asano M., Ueno Y., Nagasaki Y., Shirakawa O., Hishimoto A. Association of FKBP5 gene haplotypes with completed suicide in the Japanese population. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2011;35:252–256. doi: 10.1016/j.pnpbp.2010.11.019. [DOI] [PubMed] [Google Scholar]
- Swaab D.F., Bao A.M., Lucassen P.J. The stress system in the human brain in depression and neurodegeneration. Ageing Res. Rev. 2005;4:141–194. doi: 10.1016/j.arr.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Telese F., Gamliel A., Skowronska-Krawczyk D., Garcia-Bassets I., Rosenfeld M.G. “Seq-ing” insights into the epigenetics of neuronal gene regulation. Neuron. 2013;77:606–623. doi: 10.1016/j.neuron.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torsvall L., Akerstedt T., Lindbeck G. Effects on sleep stages and EEG power density of different degrees of exercise in fit subjects. Electroenceph. Clin. Neurophysiol. 1984;57:347–353. doi: 10.1016/0013-4694(84)90158-5. [DOI] [PubMed] [Google Scholar]
- Trapp T., Rupprecht R., Castren M., Reul J.M.H.M., Holsboer F. Heterodimerization between mineralocorticoid and glucocorticoid receptor: a new principle of glucocorticoid action in the CNS. Neuron. 1994;13:1457–1462. doi: 10.1016/0896-6273(94)90431-6. [DOI] [PubMed] [Google Scholar]
- Treves A., Rolls E.T. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Trollope A.F., Gutierrez-Mecinas M., Mifsud K.R., Collins A., Saunderson E.A., Reul J.M.H.M. Stress, epigenetic control of gene expression and memory formation. Exp. Neurol. 2012;233:3–11. doi: 10.1016/j.expneurol.2011.03.022. [DOI] [PubMed] [Google Scholar]
- van der Knaap L.J., Riese H., Hudziak J.J., Verbiest M.M., Verhulst F.C., Oldehinkel A.J., van Oort F.V. Glucocorticoid receptor gene (NR3C1) methylation following stressful events between birth and adolescence. The TRAILS study. Transl. Psychiatry. 2014;4:e381. doi: 10.1038/tp.2014.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Eekelen J.A.M., Jiang W., De Kloet E.R., Bohn M.C. Distribution of the mineralocorticoid and the glucocorticoid receptor mRNAs in the rat hippocampus. J. Neurosci. Res. 1988;21:88–94. doi: 10.1002/jnr.490210113. [DOI] [PubMed] [Google Scholar]
- van Praag H., Christie B.R., Sejnowski T.J., Gage F.H. Running enhances neurogenesis, learning and long-term potentiation in mice. Proc. Natl. Acad. Sci. U. S. A. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedder H., Weiss I., Holsboer F., Reul J.M.H.M. Glucocorticoid and mineralocorticoid receptors in rat neocortical and hippocampal brain cells in culture – characterization and regulatory studies. Brain Res. 1993;605:18–24. doi: 10.1016/0006-8993(93)91351-r. [DOI] [PubMed] [Google Scholar]
- Veldhuis H.D., De Korte C.C.M.M., De Kloet E.R. Glucocorticoids facilitate the retention of acquired immobility during forced swimming. Eur. J. Pharmacol. 1985;115:211–217. doi: 10.1016/0014-2999(85)90693-4. [DOI] [PubMed] [Google Scholar]
- Vicent G.P., Ballare C., Silvina Nacht A., Clausell J., Subtil-Rodriquez A., Quiles I., Jordan A., Beato M. Induction of progesterone target genes requires activation of Erk and Msk kinases and phosphorylation of histone H3. Mol. Cell. 2006;24:367–381. doi: 10.1016/j.molcel.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Weaver I.C., Cervoni N., Champagne F.A., D'Alessio A.C., Sharma S., Seckl J.R., Dymov S., Szyf M., Meaney M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004;7:847–854. doi: 10.1038/nn1276. [DOI] [PubMed] [Google Scholar]
- Wiegers G.J., Reul J.M.H.M. Induction of cytokine receptors by glucocorticoids: functional and pathological significance. Trends Pharmacol. Sci. 1998;19:317–321. doi: 10.1016/s0165-6147(98)01229-2. [DOI] [PubMed] [Google Scholar]
- Wilcox H.C., Storr C.L., Breslau N. Posttraumatic stress disorder and suicide attempts in a community sample of urban American young adults. Arch. Gen. Psychiatry. 2009;66:305–311. doi: 10.1001/archgenpsychiatry.2008.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willner P., Muscat R., Papp M. Chronic mild stress-induced anhedonia. A realistic animal model of depression. Neurosci. Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Willour V.L., Chen H., Toolan J., Belmonte P., Cutler D.J., Goes F.S., Zandi P.P., Lee R.S., MacKinnon D.F., Mondimore F.M., Schweizer B., DePaulo J.R., Jr., Gershon E.S., McMahon F.J., Potash J.B. Family-based association of FKBP5 in bipolar disorder. Mol. Psychiatry. 2009;14:261–268. doi: 10.1038/sj.mp.4002141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo A.P., Wrenn G., Pelletier T., Gutman A.R., Bradley B., Ressler K.J. Moderating effects of resilience on depression in individuals with a history of childhood abuse or trauma exposure. J. Affect. Disord. 2010;126:411–414. doi: 10.1016/j.jad.2010.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingo A.P., Ressler K.J., Bradley B. Resilience characteristics mitigate tendency for harmful alcohol and illicit drug use in adults with a history of childhood abuse: a cross-sectional study of 2024 inner-city men and women. J. Psychiatr. Res. 2014;51:93–99. doi: 10.1016/j.jpsychires.2014.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winokur A., Gary K.A., Rodner S., Rae-Red C., Fernando A.T., Szuba M.P. Depression, sleep physiology, and antidepressant drugs. Depress. Anxiety. 2001;14:19–28. doi: 10.1002/da.1043. [DOI] [PubMed] [Google Scholar]
- Wood G.E., Norris E.H., Waters E., Stoldt J.T., Mcewen B.S. Chronic immobilization stress alters aspects of emotionality and associative learning in the rat. Behav. Neurosci. 2008;122:282–292. doi: 10.1037/0735-7044.122.2.282. [DOI] [PubMed] [Google Scholar]
- Xie P., Kranzler H.R., Poling J., Stein M.B., Anton R.F., Farrer L.A., Gelernter J. Interaction of FKBP5 with childhood adversity on risk for post-traumatic stress disorder. Neuropsychopharmacology. 2010;35:1684–1692. doi: 10.1038/npp.2010.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalosnik M.I., Pollano A., Trujillo V., Suarez M.M., Durando P.E. Effect of maternal separation and chronic stress on hippocampal-dependent memory in young adult rats: evidence for the match–mismatch hypothesis. Stress. 2014;11:1–6. doi: 10.3109/10253890.2014.936005. [DOI] [PubMed] [Google Scholar]
- Zannas A.S., Binder E.B. Gene–environment interactions at the FKBP5 locus: sensitive periods, mechanisms and pleiotropism. Genes Brain Behav. 2014;13:25–37. doi: 10.1111/gbb.12104. [DOI] [PubMed] [Google Scholar]
- Zhou M., Bakker E.H., Velzing E.H., Berger S., Oitzl M., Joels M., Krugers H.J. Both mineralocorticoid and glucocorticoid receptors regulate emotional memory in mice. Neurobiol. Learn Mem. 2010;94:530–537. doi: 10.1016/j.nlm.2010.09.005. [DOI] [PubMed] [Google Scholar]
- Zimmermann P., Bruckl T., Nocon A., Pfister H., Binder E.B., Uhr M., Lieb R., Moffitt T.E., Caspi A., Holsboer F., Ising M. Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am. J. Psychiatry. 2011;168:1107–1116. doi: 10.1176/appi.ajp.2011.10111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zobel A., Schuhmacher A., Jessen F., Hofels S., von W.O., Metten M., Pfeiffer U., Hanses C., Becker T., Rietschel M., Scheef L., Block W., Schild H.H., Maier W., Schwab S.G. DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int. J. Neuropsychopharmacol. 2010;13:649–660. doi: 10.1017/S1461145709991155. [DOI] [PubMed] [Google Scholar]