Abstract
Objective
Inflammatory cytokines released by hypertrophic adipocytes contribute to low-grade inflammation, a characteristic of Type 2 Diabetes. Skeletal muscle contraction during physical activity stimulates the secretion of anti-inflammatory cytokines able to counteract this inflammatory status. The aim of this study was to review the evidence of the effectiveness of walking as a physical activity intervention to reduce inflammation. The interplay between adipose tissue and skeletal muscle contributions was also investigated.
Method
A structured literature review of papers available up to December 2014 was carried out within the PubMed, Scopus and ISI Web of Science databases using the keywords “walking” and “inflammation” in order to identify the studies involving healthy subjects and subjects diagnosed with, or at increased risk of, Type 2 Diabetes.
Results
Thirty-two studies were reviewed, five investigating the acute effects of walking and twenty-seven its chronic effects (n = 21 interventional and n = 6 observational). Acute effects of walking bouts led to an increase of interleukin-6 in one study, although without any increase in the concentration of the anti-inflammatory marker interleukin-1 receptor antagonist. Eight interventional studies showed a significant reduction of inflammation. A reduction in tumour necrosis factor-α concentration was often associated with an adiposity reduction. The observational studies showed that individuals who walk more present a lower inflammatory status.
Conclusion
There is no consensus regarding the efficacy of walking in the reduction of low-grade systemic inflammation, even though a relationship cannot be excluded. In each walking bout, no anti-inflammatory effect due to the IL-6-stimulated myokine cascade can be demonstrated.
Keywords: Diabetes mellitus, Type 2; Insulin resistance; Inflammation; Walking; Adipokines; Interleukin-6
Highlights
-
•
Efficacy of walking in the reduction of inflammation is controversial.
-
•
Walking efficacy in reducing inflammation is mediated by duration and intensity.
-
•
People accustomed to regular walking have lower inflammatory marker concentrations.
-
•
No anti-inflammatory effect mediated by muscular IL-6 can be demonstrated.
Introduction
Recent evidence suggests that the metabolic dysfunctions at the origin of Type 2 Diabetes (T2D) are associated with changes in the immune system, which include altered plasma levels of specific pro-inflammatory proteins and cytokines, a phenomenon known as “systemic low grade inflammation” (Duncan et al., 2003, Hotamisligil, 2006, Kolb and Mandrup-Poulsen, 2005, Schmidt et al., 1999). For this reason T2D has been classified as an inflammatory disease (Donath and Shoelson, 2011, Pradhan et al., 2001).
This inflammatory process originates in the adipose tissue as a consequence of chronic positive energy balance (excess calorie intake and/or low physical activity), which leads to adipocyte hypertrophy and macrophage infiltration (Kershaw and Flier, 2004). This scenario favours the release of “unhealthy” adipokines – the cytokines secreted by the adipose tissue – including, among others tumour necrosis factor alpha (TNF-α) and interleukin 6 (IL-6), which regulate the release of the acute phase reactant C-reactive protein (CRP) from the liver (Wellen and Hotamisligil, 2005, Zhang et al., 2006). The activation of the described inflammatory pathway appears to be linked to the onset of insulin resistance (Yudkin et al., 1999) in the peripheral tissues, namely the skeletal muscle and adipose tissue, which is an early factor of the pathogenesis of T2D (DeFronzo and Tripathy, 2009). Thus, reducing the mass of the adipose tissue seems to be the way to counteract this inflammatory status. Lifestyle modifications, such as diet and physical activity, are the elective modality to reach this goal (Arvidsson et al., 2004, Bastard et al., 2000, Bruun et al., 2006).
The recent findings that allow the skeletal muscle to be considered as an endocrine organ (Pedersen and Febbraio, 2012) have questioned the role played by IL-6 within the previously described inflammatory cascade (Febbraio et al., 2004). This stimulated new research concerning the widely recognized association between T2D, obesity and low physical activity. In fact, IL-6 has been identified as the first “myokine”, i.e. as the first cytokine secreted by skeletal muscle contraction (Steensberg et al., 2000), which induces a cascade of anti-inflammatory cytokines (Interleukin-10: IL-10 and Interleukin-1 receptor antagonist IL-1ra) (Petersen and Pedersen, 2005, Mathur and Pedersen, 2008). Thus, IL-6 seems to have a ubiquitous role (Fisman and Tenenbaum, 2010) and the relationship between its inflammatory adipokine and anti-inflammatory myokine nature has not yet been completely elucidated. This apparent paradox is possibly explained by the transient secretion of myokines, and notably IL-6, during skeletal muscle activity, which might represent one of the mechanisms blocking the inflammatory signalling pathways generated by chronically elevated levels of pro-inflammatory adipokines (Handschin and Spiegelman, 2008, Lambernd et al., 2012, Starkie et al., 2003). The inactive skeletal muscle becomes an additional source of pro-inflammatory cytokines (IL-6 and TNF-α) with respect to the adipose tissue, which contributes to enhancing the systemic inflammatory status (Handschin and Spiegelman, 2008). Considering that during contraction a skeletal muscle secretes cytokines with anti-inflammatory properties (e.g. IL-10 and IL1-ra), physical activity can potentially act through two different categories of mechanisms to control the inflammatory status: the first one entails the direct control of the source of inflammation (mainly reducing the adipose tissue mass), whereas the second one occurs during each physical activity bout through the indirect stimulation of the secretion of anti-inflammatory cytokines (Gleeson et al., 2011) from the skeletal muscle.
Walking is a suitable physical activity modality to prevent many risk factors for T2D (Murtagh et al., 2015, Qiu et al., 2014). In addition, an intervention aimed at increasing or maintaining the level of walking activity is normally easy to implement and is applicable to different populations, including obese and elderly individuals and, more broadly, people with low fitness levels, who, conversely, could encounter difficulties in performing more vigorous activities (Morris and Hardman, 1997). Whereas the effect of other types of physical activity on markers of low-grade systemic inflammation has been extensively reviewed (Hayashino et al., 2014, Hopps et al., 2011, Nimmo et al., 2013, Petersen and Pedersen, 2005), we are not aware of previous studies to date that have systematically reviewed the effect that walking can have on inflammation.
Thus, the aim of this review is to systematize the existing knowledge about the relationship between walking activity at any intensity and key markers of chronic low-grade inflammation, focusing on the implications for T2D. More specifically, the review aims to answer the following questions: 1) could walking be an appropriate physical activity for the reduction of low-grade systemic inflammation? 2) Can the potential beneficial effects of walking on the reduction of chronic low-grade inflammation originate from sources other than adipose tissue mass reduction? 3) If this is the case, can the potential beneficial effects be mediated by contracting skeletal muscles?
Methods
Eligibility criteria
This review targeted English-language studies dealing with:
-
-
Adult subjects (≥ 18 years) belonging to the following categories: a) healthy, b) having impaired glucose tolerance or metabolic syndrome and c) diagnosed with T2D;
-
-
Walking activity and inflammatory markers, as observed in randomized clinical trials, experimental, or cross-sectional studies. In particular, CRP, IL-6, and TNF-α were investigated as primary markers of low-grade inflammation, having extensively been reported to be the primary proteins and cytokines involved in the inflammatory process (Pickup, 2004, Shoelson et al., 2006).
Exclusion criteria
Studies regarding animals, including diet as lifestyle modification, or combining walking with other types of physical activity have been excluded from the analysis.
Search strategy
Articles published up to December 2014 were identified by searching on PubMed, Scopus and ISI Web of Science and by cross-reference check. The keywords used in the search are outlined in Supplementary Table 1.
Screening
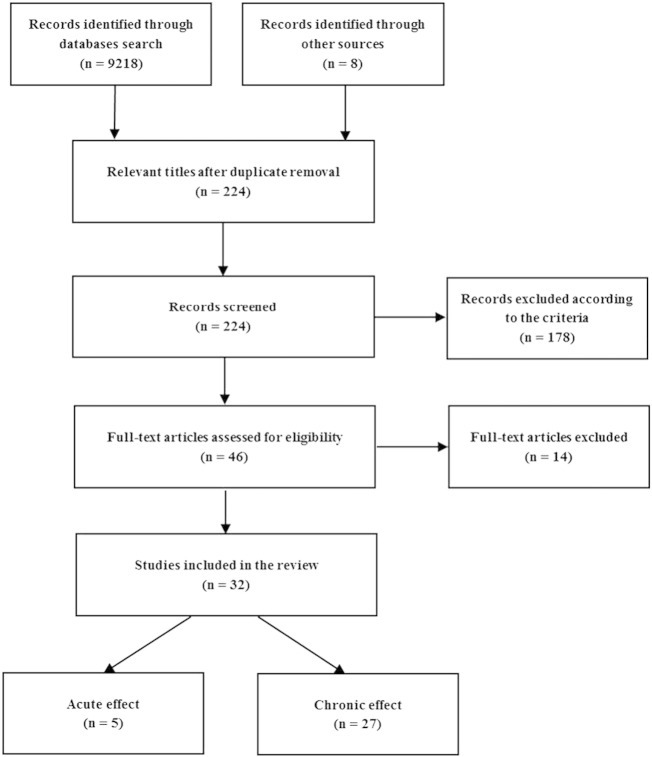
The screening process of articles by title, abstract and full article was performed based on the eligibility criteria. Fig. 1 displays the screening process of the articles.
Fig. 1.
Systematic review procedure flowchart.
Data extraction
Extracted information included year of publication, study design, objective, participant characteristics (sex, age, body mass index (BMI), and health status), sample size, outcome measurements, and assessment methodology.
Results
As a result of the above search, thirty-two articles were identified. Five of them investigated the acute response (Davis et al., 2008, Markovitch et al., 2008, Murtagh et al., 2005, Nelson and Horowitz, 2014, Nieman et al., 2005) to a walking bout and the remaining twenty-seven focused on the chronic effects of walking activity. As detailed in Table 1, twenty-one out of the twenty-seven studies regarding chronic effects were interventional (eleven randomized controlled trials and ten non-randomized trials) and six observational (five including a cross-sectional comparisons of subjects classified by activity levels and one being a cohort study).
Table 1.
Chronic effect studies: study design, method, details and outcomes.
| Author/year | Study design | Type | Method | Data analysis | Outcome related to inflammation |
|---|---|---|---|---|---|
| Balducci et al. (2010) | Randomized clinical trial. 20 out of 82 subjects (9 F and 11 M aged 63 ± 7 years; BMI 30.0 ± 1.0 kg/m2) with T2D or metabolic syndrome performed physical activity of low intensity (walking) for 12 months | Interventional | Diaries | Blood concentrations of CRP, TNF-α and IL-6 | ↘ CRP: 12% reduction from baseline observed at 12 months (not significant) ↔ BMI, waist circumference and fat mass observed at 3, 6, 9, 12 months from baseline |
| Dekker et al. (2007) | 24 middle-aged men (8 lean, 8 obese, and 8 obese with T2D) performed 60 min of walking or jogging 5 times per week (about 60% ) for 12 weeks without a reduction in body weight | Interventional | Treadmill | Blood concentrations of TNF-α, IL-6 and CRP | ↓ IL-6 concentration in all groups (52%, 32% and 17% reduction in the T2D, lean and obese groups, respectively) ↔ CRP levels ↓ Total and visceral adiposity in all groups |
| Di Raimondo et al. (2013) | 176 patients affected by metabolic syndrome (95 M and 81 F, mean age 59 ± 7 years; BMI 31.6 ± 4.8 kg/m2) completed a 24-week walking intervention (1 h/day, 5 days/week) at a walking velocity higher than the comfortable one | Interventional | Pedometers | Blood concentration of CRP before and after the intervention | ↓ BMI ↓ Waist circumference ↓ CRP |
| Dixon et al. (2013) | 9 active lean (age 52 ± 1 years; BMI 23.8 ± 0.7 kg/m2; waist circumference < 84 cm) and 9 active central overweight men (age 49 ± 1 years; BMI 29.3 ± 1.2; waist circumference > 94 cm) reduced their walking activity to less than 4000 steps/day for one week |
Interventional | Pedometers | Blood concentration of TNF-α, IL-6 and CRP before and after the intervention | ↔ TNF-α ↔ IL-6 ↔ CRP |
| Gano et al. (2011) | 11 middle-aged/older adults (5 M and 6 F, age 57–70 years; BMI 26.2 ± 1.0 kg/m2) completed a 2-month brisk walking intervention (6 days/week, 50 min/day) at 70% HRmax | Interventional | Diaries + HR monitors | Blood concentrations of TNF-α, IL-6 and CRP before and after the intervention | ↔ TNF-α ↔ IL-6 ↔ CRP ↓ BMI ↓ Total body fat |
| Giannopoulou et al. (2005) | Randomized, controlled trial, 33 women (age, 50–70 years) were assigned to diet alone (D), exercise alone (EX), or diet + exercise (D + E) for 14 weeks. The EX intervention consisted of a supervised walking programme 3 to 4 times per week for 60 min at 65% to 70% | Interventional | Supervised exercise | Blood concentrations of TNF-α, IL-6 and CRP | ↓ CRP decreased in all groups after the interventions, but no differences between groups ↘ Trend for IL-6 concentrations to decrease (P = 0.07) in all groups, with no differences between groups ↔ TNF-α ↔ BMI and total body fat in the EX group |
| Gray et al. (2009) | Randomized controlled trial. Control group (6 M, 18 F; age 51 ± 8 years; BMI 29.5 ± 5.9 kg/m2), intervention group (5 M, 19 F; age 48 ± 9 years; BMI 27.6 ± 4.9 kg/m2). 12 weeks pedometer-based walking program. Intervention is designed to increase mean daily step count by 3000 steps/day on at least 5 days of the week program | Interventional | Pedometers | Blood concentrations at baseline and after 12 weeks of TNF-α, CRP, IL-6 | ↔ TNF-α, CRP, IL-6 ↔ BMI, body fat percentage |
| Hamer and Steptoe (2008) | Cross sectional analysis including 185 healthy participants (107 M, 78 F) aged 45–59 years | Observational | Questionnaires and weekly minutes of walking | Blood concentrations of TNF-α and IL-6 | ↓ Time spent walking inversely related to TNF-α ↘ Trend observed for IL-6 to decrease |
| Ho et al. (2013) | Randomized controlled trial. 16 subjects (age range 40–66; BMI range 26–48) assigned to control group and 15 subjects (age range 44–62; BMI range 25–46) assigned to 12-week walking training at moderate intensity (30 min at 60% , 5 days/week) | Interventional | Treadmill | Blood concentration of TNF-α and IL-6 | ↓ TNF-α in overweight and obese individuals compared to no exercise ↔ IL-6 |
| Izzicupo et al. (2013) | Non randomized trial. 32 post-menopausal women (age 56 ± 4 years; BMI 26.9 ± 4.3 kg/m2) performed 13 weeks of walking training at moderate intensity (40–50 min, 4 days/week) | Interventional | Activity monitors + diaries | Plasma concentrations of TNF-α and CRP | ↓ TNF-α ↔ CRP ↔ BMI, waist circumference and fat mass percentage |
| Jennersjö et al. (2012) | Observational cross-sectional analysis including 327 individuals with T2D (224 M, 103 F; age 54–66 years). Individuals wore the pedometer for 3 days. Classification of physical activity in 4 groups | Observational | Pedometers and diaries | Blood concentrations of CRP and IL-6 | Steps/day significantly associated with lower levels of CRP and IL-6. When adjusted for waist circumference, the association between steps and IL-6 remains statistically significant but the association between steps and CRP does not |
| Klenk et al. (2013) | Population-based cohort study. Community-dwelling individuals aged over 65 underwent a baseline assessment. 710 M and 543 F (mean age 76 ± 7 years) | Observational | Accelerometer (1 week) to determine average duration of daily walking | Blood concentration of CRP at baseline | ↔ For CRP quartiles 1 and 2, no significant difference was present followed by a dose response association |
| Krause et al. (2014) | Randomized controlled trial. Twenty-five sedentary, obese (BMI > 30 kg/m2) males (53 ± 7 years); 12 controls versus 13 T2D subjects were randomly allocated to four groups that exercised for 16 weeks. Exercise consisted in 30 min/day, three times per week either at low (30–40% ) or moderate (55–65% ) intensity | Interventional | HR monitors | Blood concentrations of CRP, TNF-α and IL-6 at baseline and after 16 weeks of intervention | ↔ CRP, TNF-α, IL-6 |
| Krogh-Madsen et al. (2010) | Clinical trial. Ten healthy human males (mean age 24 ± 2 years; BMI 22.1 ± 0.7 kg/m2). None of the participants walked less than 3500 steps/day. Decrease the number of daily steps to 1500 for 14 days | Interventional | Pedometer | Blood concentrations of TNF-α and IL-6 at baseline and after 2 weeks | ↔ TNF-α, IL-6 ↓ Total body mass reduced |
| Lund et al. (2011) | Randomized controlled trial. 10 sedentary men (age 57 ± 2 years, BMI 27.9 ± 3.6 kg/m2) undertook 30 min of daily walking at 60% for 1 week versus control (normal sedentary behaviour) | Interventional | Supervised exercise | Blood concentrations of TNF-α, IL-6 and CRP | ↔ TNF-α, IL-6 and CRP |
| Marcell et al. (2005) | Randomized controlled trial. Fifty-one subjects (20 M and 31 F; BMI 33.7 ± 4.8 kg/m2) aged 45 ± 8 years were selected. No history of diabetes. Participants were randomized to 3 groups (control, moderate exercise and intense exercise) for 16 weeks of intervention. Moderate exercise was based on complete 30 min of physical activity most days of the week. All subjects were inactive at baseline. 17 subjects performed moderate exercise | Interventional | Daily diaries and supervised exercise | Blood concentration of CRP | ↔ CRP Improvements in body composition |
| McNeilly et al. (2012) | Eleven participants (6 M and 5 F; age 49 ± 9 years; BMI 32.4 ± 7.1 kg/m2) with impaired glucose tolerance, completed a 12-week brisk walking intervention (30 min/day, five days/week at 65% of HRmax) | Interventional | HR monitors + diaries | Blood concentration of CRP | ↔ CRP ↔ Dietary intake ↓ BMI and total body fat |
| Neuparth et al. (2014) | Cross-sectional study. 30 patients with T2D who walked regularly during the last year (17 M and 13 F; age 62 ± 11 years; BMI 26.6 ± 4.4 kg/m2) and 53 patients with T2D who did not perform any type of exercise (25 M and 28 F; age 63 ± 10 years; BMI 28.1 ± 3.6 kg/m2) | Observational | Questionnaires | Blood concentration of CRP | ↓ CRP levels decreased in active T2D patients with respect to inactive T2D patients, but significance lost after adjustment for BMI |
| Nishida et al. (2014) | Cross-sectional study. 737 middle-aged male subjects (age 57 ± 8 years; BMI 23.9 ± 2.9 kg/m2) and 1838 middle-aged female subjects (age 56 ± 8 years; BMI 22.6 ± 3.1 kg/m2) were monitored for 10 days to determine their physical activity level | Observational | Accelerometers | Blood concentrations of TNF-α and IL-6 | ↓ Number of steps was inversely associated with TNF-α even after adjusting for BMI ↔ IL-6 |
| Puglisi et al. (2008) | Randomized controlled trial. 12 out of 34 subjects (6 M and 6 F; age 55 ± 4 years, BMI 27.9 ± 3.9 kg/m2) assigned to a walk group for 6 week | Interventional | Pedometers | Blood concentration of TNF-α. | ↑ Daily steps for 6 week from 6000 to 11,000 steps/day ↔ TNF-α ↔ Body mass and waist circumference |
| Riesco et al. (2013) | 16 late pre-menopausal women (49 ± 3 years; BMI 31.9 ± 3.0 kg/m2) and 14 early postmenopausal (53 ± 2 years; BMI 30.8 ± 1.9 kg/m2) overweight and obese involved in 16-week walking programme (three sessions of 45 min/week at 60% of heart rate reserve). Brisk walking | Interventional | Supervised exercise | Blood concentrations of TNF-α and IL-6 | ↑ TNF-α in both groups ↔ IL-6 ↓ BMI, waist circumference and fat mass significantly reduced after adjustment for age |
| Smith et al. (2009) | Randomized controlled trial involving 41 sedentary adults (8 M, 33 F). 2 groups: 1) 16 weeks of internet delivered physical activity intervention (age 40 ± 2 years; BMI 31.4 ± 1.1 kg/m2); 2) usual care (age 47 ± 1 years; BMI 31.0 ± 0.7 kg/m2) | Interventional | Pedometer and questionnaires | Blood concentration of TNF-α and CRP | ↑ Increased mean number of steps/day by 1404 in intervention group ↔ CRP ↓ TNF-α in the intervention group after adjustment for baseline group differences ↓ Waist circumference in the intervention group after controlling for age and baseline differences |
| Taghian et al. (2012) | Randomized controlled trial involving 20 elderly women. 10 were assigned to control group (age 68 ± 4 years; BMI 31.3 ± 4.4 kg/m2) and 10 to intervention group (age 69 ± 3; BMI 28.0 ± 3.7 kg/m2). 12-week walking intervention consisting in three walking session per week for three months | Interventional | Treadmill | Blood concentration of IL-6, TNF-α and CRP | ↓ BMI, TNF-α and CRP ↔ IL-6 |
| Yakeu et al. (2010) | 17 healthy sedentary individuals (9 M and 8 F of mean age 46 ± 11 years, mean BMI 26.8 ± 5.1 kg/m2) undertook an 8-week low-intensity exercise programme (walking 10,000 steps/day, three times/week). | Interventional | Supervised exercise | Plasma concentration of IL-6 and IL-10 | ↓ IL-6 decreased 33% within 4 weeks ↑ IL-10 ↔ BMI |
| Yates et al. (2008) | Cross-sectional study including 400 participants normal, with pre-diabetes, with diabetes (mean age 62 ± 9 years) | Observational | Questionnaires | Blood concentrations of IL-6, TNF-α and CRP | ↓ IL-6, CRP and TNF-α level in group reporting walking for at least 30 min on at least 5 days/week compared group reporting lower walking activity, after adjustment for potential confounders. Further adjustment for waist circumference attenuated the association of walking with TNFα, although the association with IL-6 and CRP remained significant. |
| Yates et al. (2010) | Randomized controlled trial including 74 participants (age 65 ± 8 years) with impaired glucose tolerance and BMI over 25. 3 groups: 1) pedometer; 2) without pedometer; 3) usual care. 12 months |
Interventional | Pedometer and questionnaire | Blood concentrations of IL-6 and CRP at baseline and after 12 months | ↔ IL-6 and CRP Ambulatory activity was significantly and inversely associated with IL-6 after adjustment for potential confounders (age, ethnicity, sex, group, medication status, baseline and change in BMI) |
| Zoppini et al. | 16 sedentary (8 M and 8 F), overweight (BMI 29.0 ± 3.0 kg/m2), non-smoking, older patients (age 66 ± 6 years) with T2D volunteered to participate in a 6-month, supervised, progressive, aerobic training study, two times per week | Interventional | Supervised exercise | Plasma levels of CRP and TNF-α at baseline and after 6 months | ↔ CRP and TNF-α ↔ BMI, waist circumference and body weight |
Abbreviations: M: male; F: female; T2D: Type 2 Diabetes; CRP: C-Reactive Protein; TNF-α: Tumour Necrosis Factor α; IL-6: Interleukin 6; : Maximal Oxygen Uptake; BMI: Body Mass Index.
Symbols: ↑ Significant increase; ↗ Trend to increase (not significant); ↔ No variation; ↘ Trend to decrease (not significant); ↓ Significant decrease.
Acute response to walking bouts
One of the five studies dealing with the acute effects of walking (Markovitch et al., 2008) involved twelve sedentary male volunteers (age 54 ± 4 years; BMI 28.0 ± 3.0 kg/m2) who underwent a single walking bout of moderate intensity (30 min at 50% of maximal oxygen uptake (), estimated intensity of 5.2 ± 0.7 METs (METs, multiple of energy consumption at rest)). IL-6, IL-10, and CRP concentrations did not change at 0, 2, 24, 48, 72, and 168 h following the trial. Similarly, no significant differences were found in plasma CRP when twelve sedentary healthy post-menopausal women (age 58 ± 6 years; BMI 25.6 ± 3.4 kg/m2) completed two 30-minute treadmill brisk walking exercises (50% and 70% of their maximal heart rate (HRmax), corresponding to 35% and 60% estimated , respectively), (Davis et al., 2008), or in fifteen obese male subjects (age 50 ± 6 years; BMI 30.9 ± 4.9 kg/m2) after a 45-minute walking at 60% (Murtagh et al., 2005).
A slight but significant increase in serum IL-6 concentration, not associated with changes in IL-1ra concentration, was observed 1 h after a 30-minute intervention consisting of treadmill walking at 60–65% in fifteen women (age 37 ± 3 years; non-obese) who were accustomed to regular walking (Nieman et al., 2005). In a later trial (Nelson and Horowitz, 2014), IL-6 and TNF-α concentrations remained unchanged one day after a session of exercise (1 h of treadmill brisk walking at 70% of HRmax) performed by twenty-four healthy subjects, twelve (male/female: 5/7; age 27 ± 2 years; BMI 30.9 ± 1.0 kg/m2) of which were “regular-exercisers” and twelve (male/female: 5/7; age 28 ± 2 years; BMI 30.1 ± 0.5 kg/m2) “non-exercisers”.
It has to be highlighted that the study by Nelson and Horowitz (2014) has been included among those on the acute effects for the purpose of this review despite the fact that IL-6 was measured one day after the walking bout. From the other studies, however, it might be deduced that walking bouts do not seem to activate the IL-6 based anti-inflammatory pathway during or directly after a walking bout. Indeed, a non-significant reduction of IL-6 concentration with respect to the pre-exercise level has been reported by Murtagh et al. (2005).
Nieman et al. (2005) reported an increase in IL-6, but no increment in plasma concentration of the anti-inflammatory marker IL-1ra. As expected, no investigation of TNF-α was found among the reviewed studies. TNF-α, in fact, is mainly secreted by the adipose tissue and does not seem related to the cytokine cascade triggered by skeletal muscle contraction. None of the reviewed studies reported acute variation in the level of CRP in association to a walking intervention.
Chronic effects of walking activity
The methods generally adopted for the assessment of physical activity performed in free-living conditions, that is, non-supervised by an expert, can be summarized as self-reported (e.g. questionnaires and diaries) or objective measures based on wearable sensors (e.g. accelerometers, pedometers, heart rate monitors).
Out of the twenty-seven reviewed studies regarding the chronic effect of walking, eighteen dealt with free-living conditions (see Table 1). Four of those used self-reporting assessment tools (Balducci et al., 2010, Hamer and Steptoe, 2008, Neuparth et al., 2014, Yates et al., 2008), and eight used objective measures (five used pedometers (Di Raimondo et al., 2013, Dixon et al., 2013, Gray et al., 2009, Krogh-Madsen et al., 2010, Puglisi et al., 2008), two used accelerometers (Klenk et al., 2013, Nishida et al., 2014) and one used heart rate monitors (Krause et al., 2014)). The remaining six studies used a combination of self-reporting and objective assessment (Gano et al., 2011, Izzicupo et al., 2013, Jennersjö et al., 2012, McNeilly et al., 2012, Smith et al., 2009, Yates et al., 2010). The use of objective measurement methods is preferred over the use of self-reporting methods because, as widely recognized, the latter are affected by reporting biases (Prince et al., 2008), which may undermine the accurate classification and quantification of physical activity.
Interventional studies
Reviewed studies using walking as physical activity intervention modality have produced conflicting results. Only eight out of twenty-one reported a significant reduction of chronic low-grade inflammation marker concentration (Dekker et al., 2007, Di Raimondo et al., 2013, Giannopoulou et al., 2005, Ho et al., 2013, Izzicupo et al., 2013, Smith et al., 2009, Taghian et al., 2012, Yakeu et al., 2010).
In ten sedentary overweight men (age 57 ± 2 years; BMI 27.9 ± 3.6 kg/m2), one week of 30-minute daily walking at 60% did not elicit any significant change in TNF-α, IL-6 or CRP with respect to the baseline values (Lund et al., 2011). In addition, no significant differences were found in TNF-α, IL-6 or CRP plasma concentrations in eleven subjects (age 57–70 years, BMI 26.2 ± 1.0 kg/m2) tested before and after a two-month brisk walking intervention (6 days/week, 50 min/day at 70% HRmax equivalent to 60% ) (Gano et al., 2011) or in twenty-five subjects (twelve obese controls vs. thirteen obese with T2D, age 53 ± 7 years) that exercised for sixteen weeks (30 min/day, three times per week either at low, 30–40% , or moderate, 55–65% , intensity) (Krause et al., 2014). A significant change in body composition (BMI and total body fat) was highlighted only by Gano et al. (2011). No difference in CRP concentration was observed in eleven obese subjects (age 49 ± 9 years; BMI 32.4 ± 7.1 kg/m2) performing a twelve-week intervention consisting of 30 min/day of brisk walking, 5 days/week at 65% HRmax (~ 50–60% ) (McNeilly et al., 2012), despite the same intervention being sufficient to lower BMI and total body fat, without changes in dietary intake.
One week of reduced physical activity (steps/day < 4000) induced no changes in IL-6, CRP and TNF-α in nine healthy active lean (age 52 ± 1 years; BMI 23.8 ± 0.7 kg/m2; waist circumference < 84 cm) and nine healthy active central overweight men (age 49 ± 1 years; BMI 29.3 ± 1.2 kg/m2; waist circumference > 94 cm) (Dixon et al., 2013). A reduction of walking from 10,000 steps/day to 1500 steps/day for fourteen days did not lead to differences in TNF-α and IL-6 in ten healthy males (age 24 ± 2 years; BMI 22.1 ± 0.7 kg/m2), despite an attenuation in insulin sensitivity (Krogh-Madsen et al., 2010). Surprisingly, the subjects showed a reduction in total body mass.
Consistent results have been reported for a walking intervention involving twenty-four healthy subjects (age 48 ± 9 years; BMI 27.6 ± 4.9 kg/m2) which aimed to gradually increase the average number of daily steps walked by the participants (Gray et al., 2009). The subjects were observed for one week, to set a baseline value. They were then asked to achieve the goal of walking more than 3000 steps above that baseline value, for at least five days within the next six weeks, and then to maintain this increase for other six weeks. The results showed that this twelve-week walking programme did not modify TNF-α, IL-6 and CRP levels in addition to BMI and body fat percentage when compared to the control group of twenty-four healthy subject (age 51 ± 8 years; BMI 29.5 ± 5.9 kg/m2). A similar study including twelve subjects (age 55 ± 4; BMI 27.9 ± 3.9 kg/m2) did not show any modification in TNF-α concentration from the baseline after a six-week intervention, which led to an increase in steps/day from 6000 to 11,000 (Puglisi et al., 2008). In this study, no modification of body mass or waist circumference was detected.
A further walking intervention protocol prescribed 60 min of walking at 60% , five times per week for twelve weeks, to twenty-four middle aged subjects (Dekker et al., 2007) who also followed a dietary programme designed to maintain body mass. The intervention led to a reduction of IL-6 levels, visceral and total adiposity. This reduction was significant even when the subjects were divided into three sub-groups: lean, obese and obese with T2D (age 48 ± 3 years, 47 ± 3 years and 51 ± 3 years, respectively). The intervention, however, did not lead to significantly altered CRP levels. A consistent reduction of IL-6 was also detected when seventeen healthy adults (age 46 ± 11 years, BMI 26.8 ± 5.1 kg/m2) performed an eight-week low-intensity exercise intervention consisting of walking 10,000 steps/day (Yakeu et al., 2010). The IL-6 concentration decreased to approximately two-thirds of its basal level within four weeks of beginning the exercise. At the same time, the IL-10 significantly increased and no change in BMI was observed.
A significant decrease in BMI and fat percentage, associated with a decrease in TNF-α and CRP and no changes in IL-6, were detected in a randomized trial including twenty elderly women. The subjects were assigned to either a control group (age 68 ± 4 years; BMI 31.3 ± 4.4 kg/m2) or an intervention group (age 69 ± 3 years; BMI 28.0 ± 3.7 kg/m2) who underwent a twelve-week walking programme (three walking sessions per week, the duration and intensity of which increased from 20 min at 50–55% HR reserve, ~ 50–55% , to 45–60 min at 60–70% HR reserve, ~ 60–70% , in the last week) (Taghian et al., 2012).
A further randomized trial (Ho et al., 2013), compared two groups of sedentary and lightly active healthy subjects to a twelve-week intervention group (control: age 40–66 years, BMI between 26.0 and 48.0 kg/m2; intervention: age 44–62 years, BMI between 25.0 and 45.6 kg/m2). The intervention consisted of 30-minute treadmill walking at 60% of HR reserve (~ 60% ) for 5 days a week. The TNF-α levels were decreased by 20% at week 12 compared to the baseline in the intervention group. These changes in TNF-α significantly correlated with a change in body fat and body fat percentage. No significant changes were observed for the IL-6 within or between groups.
The finding of a 24-week walking intervention study (1 h/day, 5 days/week) involving 176 patients with metabolic syndrome (mean age 59 ± 8 years; BMI 31.6 ± 4.8 kg/m2) (Di Raimondo et al., 2013), for whom the mean number of steps walked per day was less than 10,000 was a decrease in BMI associated with a decrease in CRP concentration. In contrast, another sixteen-week walking programme (three session of 45 min/week at ~ 60% ) did not modify IL-6 and CRP levels but increased plasma levels of TNF-α in sixteen late pre-menopausal (age 49 ± 3 years; BMI 31.9 ± 3.0 kg/m2) and fourteen early post-menopausal (age 53 ± 2 years; BMI 30.8 ± 1.9 kg/m2) overweight or obese women (Riesco et al., 2013). In both groups, the intervention led to statistically significant differences in BMI, waist circumference and fat mass, after adjustment for age. Intriguingly, a thirteen-week intervention (four walking sessions of 40–50 min at moderate intensity) in thirty-two post-menopausal women (mean age 56 ± 4 years; BMI 26.9 ± 4.3 kg/m2), showed the same results in terms of CRP, a significant reduction of TNF-α but a non-significant variation of BMI and fat mass (Izzicupo et al., 2013).
A sixteen-week intervention duration was used in a further study (Marcell et al., 2005) where fifty-one inactive overweight and obese individuals with no history of diabetes were randomized in three groups. Out of these, seventeen (age 47 ± 9 years; BMI 33.9 ± 4.9 kg/m2) underwent a moderate walking intervention (3.5 METS of intensity per session), which improved their body composition, with decreased body weight, total body fat, and BMI. However, no change in CRP levels was found following this intervention. Conversely, another programme (fourteen weeks of sixty minutes walking at 60% three or four times per week) involving eleven overweight and obese post-menopausal women with T2D (age 56 ± 2 years; BMI 35.9 ± 1.9 kg/m2) led to a significant decrease in CRP levels (Giannopoulou et al., 2005). IL-6 plasma concentration tended to decrease, but no significant differences from the baseline were reported. Furthermore, no significant differences were observed in TNF-α, BMI or total body fat either. In a randomized trial (Smith et al., 2009) nineteen sedentary adults (age 40 ± 2 years; BMI 31.4 ± 1.1 kg/m2) were assigned to sixteen weeks of walking intervention. An increase of 1404 steps/day caused no effect on CRP levels, whereas TNF-α declined significantly. When adjusting for age and baseline differences, waist circumference decreased in the intervention group as well.
A group of sixteen sedentary, overweight, non-smoking, older patients with T2D (age 66 ± 6 years; BMI 29.0 ± 3.0 kg/m2) participated in a six-month, twice a week, supervised 60-minute aerobic training intervention (50% and 70% of HR reserve ~ 50–70% ) (Zoppini et al., 2006). TNF-α, CRP, body weight and waist circumference did not change after the intervention. Twenty subjects (age 63 ± 7 years; BMI 30.0 ± 1.0 kg/m2) with either T2D or metabolic syndrome performed a low intensity walking activity for twelve months, which caused a non-significant decrease of 12% in CRP levels (Balducci et al., 2010). No differences from the baseline values were found in IL-6, TNF-α and BMI. Another study (Yates et al., 2010) involving two groups of twenty-four overweight subjects with impaired glucose tolerance failed to show reductions on markers of chronic low grade inflammation after twelve months of intervention with (age 65 ± 8 years; BMI 28.7 ± 5.0 kg/m2) and without pedometer (age 64 ± 7 years; BMI 29.3 ± 5.1 kg/m2). However, across the study sample, a change in objectively measured walking activity was significantly and inversely associated with IL-6, after adjustment for potential confounders, including changes in BMI. Notably, a variation of ambulatory activity by about 2500 steps⁄day, equivalent to approximately 25 min of moderate-intensity walking activity per day, was needed to induce a 0.5 pg⁄ml decrease in IL-6 (Tudor-Locke and Bassett, 2004).
Observational studies
Reviewed observational studies provided a more univocal inverse relationship between habitual walking activity and markers of low-grade systemic inflammation. A cross-sectional study (Yates et al., 2008) including 400 participants (age 62 ± 9 years) screened for T2D (normal, pre-diabetic and diabetic) showed that those who reported walking activity for at least 30 min on at least 5 days/week had lower levels of CRP, IL-6, and TNF-α compared to those who reported lower walking activity levels. This was after adjustment for other types of moderate to vigorous intensity physical activity, age, ethnicity, sex, social deprivation and smoking status. Further adjustment for waist circumference attenuated the association of walking with TNF-α. In another cross-sectional study (Hamer and Steptoe, 2008) on 185 healthy subjects aged between 45 and 59, multiple linear regression analyses adjusted for all confounders, revealed that time spent walking (min/week) is inversely related to TNF-α while a non-significant trend was observed for IL-6.
A more recent cross-sectional study (Jennersjö et al., 2012) involving 327 individuals (aged 54–66 years) with T2D aimed to classify, by pedometer count, four different levels of physical activity (< 5000 steps/day; 5000–7499 steps/day; 7500–9999 steps/day and > 10,000 steps/day). This study showed a significant negative correlation between the number of steps/day and CRP and IL-6 levels. When adjusting for waist circumference, the association between IL-6 and steps⁄day remained statistically significant, whereas the association between high-sensitivity CRP and steps⁄day did not reach statistical significance. In a cross-sectional study by Nishida et al. (2014) involving 1838 individuals aged 40 to 69 years, habitual physical activity was assessed by a single axis accelerometer. Step count was inversely associated with TNF-α even after adjusting for BMI, whereas no significant differences were observed in IL-6. In another cross-sectional study (Neuparth et al., 2014) data from thirty patients (age 62 ± 11 years; BMI 26.6 ± 4.4 kg/m2) with T2D who walked regularly (from 30 min up to 1 h a day, 3 to 5 times per week) during the last year and fifty-three patients (age 63 ± 10 years; BMI 28.1 ± 3.6 kg/m2) with T2D who did not perform any type of exercise were compared. Active T2D patients showed significantly lower BMI and CRP levels than inactive T2D patients, even if CRP lost significance after adjustment for BMI.
Finally, one cohort study was found, which involved 1253 community-dwelling individuals (age 76 ± 7 years) (Klenk et al., 2013). Stratification for CRP quartiles after adjustment for covariates, highlighted that an increase of 10 min per day of walking duration can elicit significant reduction of CRP levels.
Discussion
The first aim of this review was to assess whether walking can be an appropriate physical activity intervention to reduce low-grade systemic inflammation. The investigated interventional studies provided controversial results. Only eight (Dekker et al., 2007, Di Raimondo et al., 2013, Giannopoulou et al., 2005, Ho et al., 2013, Izzicupo et al., 2013, Smith et al., 2009, Taghian et al., 2012, Yakeu et al., 2010) out of twenty-one reported a significant reduction of chronic low-grade inflammation, intended as the reduction of the concentration of at least one of the investigated markers, namely CRP, IL-6 and TNF-α. Although the number of studies showing a reduction in the low-grade systemic inflammation after a walking intervention is considerably lower than those reporting no changes, the efficacy of walking as a way to reduce low-grade inflammation cannot be excluded. The observational studies, in fact, unequivocally showed that individuals who usually walk more have lower IL-6, TNF-α and CRP concentrations and some, comparatively large sample, interventional and observational studies (Di Raimondo et al., 2013, Nishida et al., 2014, Smith et al., 2009) supported the efficacy of walking in reducing the inflammatory status.
In an attempt to identify common patterns in the studies supporting the efficacy of walking, it has been observed that impaired glucose tolerance or T2D does not seem to be particularly relevant, whereas fat mass plays a key role. Evidence in the literature also suggests that the increased circulating IL-6 concentrations seen in patients with T2D are strongly related to fat mass but not to insulin responsiveness, suggesting that neither IL-6 nor TNFα are indicators of insulin resistance (Carey et al., 2004). Moreover, screening for body weight, the reviewed studies involving overweight or obese subjects that showed a reduction in TNF-α plasma concentration after the intervention, often reported a concomitant reduction of adiposity (Ho et al., 2013, Smith et al., 2009, Taghian et al., 2012). This result is also in accordance with the study by Hotamisligil et al. (1995), demonstrating that body weight reduction in obese subjects is associated with a decrease in TNF-α mRNA expression in fat tissue.
The duration of the intervention represents one of the major determinants of the efficacy of a walking intervention. In fact, even intense interventions lasting one or two weeks (Dixon et al., 2013, Krogh-Madsen et al., 2010, Lund et al., 2011) did not cause modifications of any of the observed inflammatory markers, despite being potentially effective in modifying other markers of the metabolic system status, such as peripheral insulin sensitivity (Krogh-Madsen et al., 2010). The shortest among the reviewed intervention studies that induced a low-grade inflammation variation is an eight-week intervention (Yakeu et al., 2010). The critical role of intervention duration has also been described by Hayashino et al. (2014), who performed a meta-analysis of fourteen randomized controlled trials involving different types of exercise (aerobic, resistance or a combination of both). This meta-analysis highlighted that exercise was more effective in reducing IL-6 in longer interventions, which included a larger number of sessions. Notably, a reduction of low-grade inflammation has been detected only in studies where walking activity was supervised or objectively monitored (Dekker et al., 2007, Di Raimondo et al., 2013, Giannopoulou et al., 2005, Ho et al., 2013, Izzicupo et al., 2013, Smith et al., 2009, Taghian et al., 2012, Yakeu et al., 2010), thus indicating that a bias could affect self-reported quantification of physical activity and hence the final outcome. The intensity of walking activity can be hypothesized as a further determinant. Most of the reviewed studies that reported a reduction of inflammation are characterized by a moderate-intensity walking activity (Dekker et al., 2007, Giannopoulou et al., 2005, Ho et al., 2013, Izzicupo et al., 2013, Taghian et al., 2012), confirming the well-known beneficial effects of brisk walking in T2D (American Diabetes Association, 2014, Jeon et al., 2007).
From the literature analysis the efficacy of walking in the reduction of the inflammatory status cannot be excluded, the review therefore aimed to further investigate the mechanisms by which this potential effect is exerted. In this context, the authors wanted to assess if the beneficial effects of walking with regards to the reduction of chronic low-grade inflammation originate from sources different from adipose tissue mass reduction and, in particular, whether the skeletal muscles play a role in this context. A key point to test this hypothesis is to determine if walking induces the same anti-inflammatory reactions induced by vigorous exercise, possibly in a less marked fashion. Despite the fact that IL-6 has been shown to increase up to 100 times as a function of intensity and duration of skeletal muscles contractions (Pedersen and Febbraio, 2008), only one out of the five reviewed studies regarding the acute effect of walking (Davis et al., 2008, Markovitch et al., 2008, Murtagh et al., 2005, Nelson and Horowitz, 2014, Nieman et al., 2005) reported a slight increase one hour after the walking session (Nieman et al., 2005). Surprisingly, the only population showing an increase of IL-6 consisted of women accustomed to regular walking (Nieman et al., 2005). As is well known, differences in initial training level determine the magnitude of exercise-induced IL-6 responses, most likely due to differences in muscle glycogen content between trained and untrained skeletal muscles (Heinrich et al., 1990, Phillips et al., 1996). During acute exercise, the untrained muscle is highly dependent on glycogen substrate and acute plasma IL-6 response is lower in trained than untrained subjects (Pedersen and Febbraio, 2008). For this reason, individuals who are not accustomed to regular walking should show a wider IL-6 response. Moreover, no increment was detected in the anti-inflammatory cytokine IL1-ra by Nieman et al. (2005). Thus, at present, no acute effect caused by an IL-6-stimulated anti-inflammatory cascade can be demonstrated. However, no definitive conclusion should be drawn, considering the limited number of available studies.
A key role in the reduction of low-grade inflammation could be played by the peroxisome proliferator-activated receptor γ co-activator 1α (PGC1-α), which is expressed in the skeletal muscle. An increase in PGC1-α expression is one of the main adaptations due to chronic exercise. PGC1-α suppresses a broad inflammatory response, controls muscle plasticity, and mediates some of the beneficial effects of the exercise (Handschin and Spiegelman, 2008). However, recent evidence showed that PGC-1-α expression in the skeletal muscle depends on the exercise intensity, being evident only above the lactate threshold (Tobina et al., 2011). These findings confirm that the intensity of walking could be one of the major factors toward inflammation reduction.
It is important to underline that our results are strongly limited by the heterogeneity in the study design. In addition, nutritional regimes play a key role in the investigated context, and might have been a possible confounding factor. Furthermore, the different methods to assess physical activity in free-living context make the comparison of results difficult: further studies using objective assessment methods of walking activity are encouraged. Moreover, the role played by biological inter- and intra-individual measurement variability (Navarro et al., 2012) in markers of chronic low-grade inflammation should be investigated further. Last but not least, further inflammatory markers that could have a role in describing low-grade inflammation (e.g. IL-10, adiponectin, leptin) were not analysed in this work but could be the object of further investigations.
Conclusion
No consensus regarding the efficacy of walking in the reduction of low-grade systemic inflammation appeared from this review, even though its role cannot be excluded. The duration and intensity of the intervention influence the skeletal muscle adaptations that determine the final outcome. It is plausible to conclude that the reduction of adiposity mediates the reduction of inflammation, whereas the anti-inflammatory effect elicited by IL-6 cannot be confirmed. The level of glucose tolerance does not seem to influence the final outcome. An objective assessment in wider population studies, analysing the role played by different duration and intensity could facilitate the definition of a dose–response relationship that could be useful in defining a model of the inflammatory process (Castiglione et al., 2013).
Conflict of interest statement
The authors declare that there are no conflicts of interests.
Acknowledgments
This study was funded by the European Commission under the 7th Framework Programme (MISSION-T2D project, contract no. 600803). The funding agency (European Commission) had no role in the study design, in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. The contribution of Clare Sansom in the editing of the paper is gratefully acknowledged.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.pmedr.2015.06.012.
Appendix A. Supplementary data
Supplementary material: specific search terms.
References
- American Diabetes Association Standards of medical care in diabetes — 2014. Diabetes Care. 2014;37(Suppl. 1):S14–S80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- Arvidsson E., Viguerie N., Andersson I., Verdich C., Langin D., Arner P. Effects of different hypocaloric diets on protein secretion from adipose tissue of obese women. Diabetes. 2004;53:1966–1971. doi: 10.2337/diabetes.53.8.1966. [DOI] [PubMed] [Google Scholar]
- Balducci S., Zanuso S., Nicolucci A., Fernando F., Cavallo S., Cardelli P., Fallucca S., Alessi E., Letizia C., Jimenez A., Fallucca F., Pugliese G. Anti-inflammatory effect of exercise training in subjects with type 2 diabetes and the metabolic syndrome is dependent on exercise modalities and independent of weight loss. Nutr. Metab. Cardiovasc. Dis. 2010;20(8):608–617. doi: 10.1016/j.numecd.2009.04.015. [DOI] [PubMed] [Google Scholar]
- Bastard J.P., Jardel C., Bruckert E., Blondy P., Capeau J., Laville M., Vidal H., Hainque B. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. J. Clin. Endocrinol. Metab. 2000;85:3338–3342. doi: 10.1210/jcem.85.9.6839. [DOI] [PubMed] [Google Scholar]
- Bruun J.M., Helge J.W., Richelsen B., Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am. J. Physiol. Endocrinol. Metab. 2006;290(5):E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- Carey A.L., Bruce C.R., Sacchetti M., Anderson M.J., Olsen D.B., Saltin B., Hawley J.A., Febbraio M.A. 6. Vol. 47. 2004. Interleukin-6 and tumor necrosis factor-alpha are not increased in patients with Type 2 diabetes: evidence that plasma interleukin-6 is related to fat mass and not insulin responsiveness; pp. 1029–1037. [DOI] [PubMed] [Google Scholar]
- Castiglione F., Tieri P., De Graaf A., Franceschi C., Liò P., Van Ommen B., Mazzà C., Tuchel A., Bernaschi M., Samson C., Colombo T., Castellani G.C., Capri M., Garagnani P., Salvioli S., Nguyen V.A., Bobeldijk-Pastorova I., Krishnan S., Cappozzo A., Sacchetti M., Morettini M., Ernst M. The onset of type 2 diabetes: proposal for a multi-scale model. JMIR Res. Protocol. 2013;2(2):e44. doi: 10.2196/resprot.2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis J., Murphy M., Trinick T., Duly E., Nevill A., Davison G. Acute effects of walking on inflammatory and cardiovascular risk in sedentary post-menopausal women. J. Sports Sci. 2008;26(3):303–309. doi: 10.1080/02640410701552906. [DOI] [PubMed] [Google Scholar]
- DeFronzo R.A., Tripathy D. Skeletal muscle insulin resistance is the primary defect in type 2 diabetes. Diabetes Care. 2009;32(Suppl. 2):S157–S163. doi: 10.2337/dc09-S302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekker M.J., Lee S., Hudson R., Kilpatrick K., Graham T.E., Ross R., Robinson L.E. An exercise intervention without weight loss decreases circulating interleukin-6 in lean and obese men with and without type 2 diabetes mellitus. Metabolism. 2007;56(3):332–338. doi: 10.1016/j.metabol.2006.10.015. [DOI] [PubMed] [Google Scholar]
- Di Raimondo D., Tuttolomondo A., Buttà C., Casuccio A., Giarrusso L., Miceli G., Licata G., Pinto A. Metabolic and anti-inflammatory effects of a home-based programme of aerobic physical exercise. Int. J. Clin. Pract. 2013;67(12):1247–1253. doi: 10.1111/ijcp.12269. [DOI] [PubMed] [Google Scholar]
- Dixon N.C., Hurst T.L., Talbot D.C., Tyrrell R.M., Thompson D. Effect of short-term reduced physical activity on cardiovascular risk factors in active lean and overweight middle-aged men. Metabolism. 2013;62(3):361–368. doi: 10.1016/j.metabol.2012.08.006. [DOI] [PubMed] [Google Scholar]
- Donath M.Y., Shoelson S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011;11(2):98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- Duncan B.B., Schmidt M.I., Pankow J.S., Ballantyne C.M., Couper D., Vigo A., Hoogeveen R., Folsom A.R., Heiss G. Low-grade systemic inflammation and the development of type 2 diabetes: the atherosclerosis risk in communities study. Diabetes. 2003;52(7):1799–1805. doi: 10.2337/diabetes.52.7.1799. [DOI] [PubMed] [Google Scholar]
- Febbraio M.A., Hiscock N., Sacchetti M., Fischer C.P., Pedersen B.K. Interleukin-6 is a novel factor mediating glucose homeostasis during skeletal muscle contraction. Diabetes. 2004;53(7):1643–1648. doi: 10.2337/diabetes.53.7.1643. [DOI] [PubMed] [Google Scholar]
- Fisman E.Z., Tenenbaum A. The ubiquitous interleukin-6: a time for reappraisal. Cardiovasc. Diabetol. 2010;9:62. doi: 10.1186/1475-2840-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gano L.B., Donato A.J., Pierce G.L., Pasha H.M., Magerko K.A., Roeca C., Seals D.R. Increased proinflammatory and oxidant gene expression in circulating mononuclear cells in older adults: amelioration by habitual exercise. Physiol. Genomics. 2011;43(14):895–902. doi: 10.1152/physiolgenomics.00204.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giannopoulou I., Fernhall B., Carhart R., Weinstock R.S., Baynard T., Figueroa A., Kanaley J.A. Effects of diet and/or exercise on the adipocytokine and inflammatory cytokine levels of postmenopausal women with type 2 diabetes. Metabolism. 2005;54(7):866–875. doi: 10.1016/j.metabol.2005.01.033. [DOI] [PubMed] [Google Scholar]
- Gleeson M., Bishop N.C., Stensel D.J., Lindley M.R., Mastana S.S., Nimmo M.A. The anti-inflammatory effects of exercise: mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011;11:607–615. doi: 10.1038/nri3041. [DOI] [PubMed] [Google Scholar]
- Gray S.R., Baker G., Wright A., Fitzsimons C.F., Mutrie N., Nimmo M.A. The effect of a 12 week walking intervention on markers of insulin resistance and systemic inflammation. Prev. Med. 2009;48(1):39–44. doi: 10.1016/j.ypmed.2008.10.013. [DOI] [PubMed] [Google Scholar]
- Hamer M., Steptoe A. Walking, vigorous physical activity, and markers of hemostasis and inflammation in healthy men and women. Scand. J. Med. Sci. Sports. 2008;18(6):736–741. doi: 10.1111/j.1600-0838.2007.00747.x. [DOI] [PubMed] [Google Scholar]
- Handschin C., Spiegelman B.M. The role of exercise and PGC1alpha in inflammation and chronic disease. Nature. 2008;454(7203):463–469. doi: 10.1038/nature07206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashino Y., Jackson J.L., Hirata T., Fukumori N., Nakamura F., Fukuhara S., Tsujii S., Ishii H. Effects of exercise on C-reactive protein, inflammatory cytokine and adipokine in patients with type 2 diabetes: a meta-analysis of randomized controlled trials. Metabolism. 2014;63(3):431–440. doi: 10.1016/j.metabol.2013.08.018. [DOI] [PubMed] [Google Scholar]
- Heinrich P.C., Castell J.V., Andus T. Interleukin-6 and the acute phase response. Biochem. J. 1990;265(3):621–636. doi: 10.1042/bj2650621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho S.S., Dhaliwal S.S., Hills A.P., Pal S. Effects of chronic exercise training on inflammatory markers in Australian overweight and obese individuals in a randomized controlled trial. Inflammation. 2013;36(3):625–632. doi: 10.1007/s10753-012-9584-9. [DOI] [PubMed] [Google Scholar]
- Hopps E., Canino B., Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011;48(3):183–189. doi: 10.1007/s00592-011-0278-9. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444(7121):860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Arner P., Caro J.F., Atkinson R.L., Spiegelman B.M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J. Clin. Invest. 1995;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzicupo P., D'Amico M.A., Bascelli A., Di Fonso A., D'Angelo E., Di Blasio A., Bucci I., Napolitano G., Gallina S., Di Baldassarre A. Walking training affects dehydroepiandrosterone sulfate and inflammation independent of changes in spontaneous physical activity. Menopause. 2013;20(4):455–463. doi: 10.1097/gme.0b013e31827425c9. [DOI] [PubMed] [Google Scholar]
- Jennersjö P., Ludvigsson J., Länne T., Nystrom F.H., Ernerudh J., Östgren C.J. Pedometer-determined physical activity is linked to low systemic inflammation and low arterial stiffness in Type 2 diabetes. Diabet. Med. 2012;29(9):1119–1125. doi: 10.1111/j.1464-5491.2012.03621.x. [DOI] [PubMed] [Google Scholar]
- Jeon C.Y., Lokken R.P., Hu F.B., Van Dam R.M. Physical activity of moderate intensity and risk of type 2 diabetes: a systematic review. Diabetes Care. 2007;30(3):744–752. doi: 10.2337/dc06-1842. [DOI] [PubMed] [Google Scholar]
- Kershaw E.E., Flier J.S. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Klenk J., Denkinger M., Nikolaus T., Peter R., Rothenbacher D., Koenig W. Association of objectively measured physical activity with established and novel cardiovascular biomarkers in elderly subjects: every step counts. J. Epidemiol. Community Health. 2013;67(2):194–197. doi: 10.1136/jech-2012-201312. [DOI] [PubMed] [Google Scholar]
- Kolb H., Mandrup-Poulsen T. An immune origin of type 2 diabetes? Diabetologia. 2005;48(6):1038–1050. doi: 10.1007/s00125-005-1764-9. [DOI] [PubMed] [Google Scholar]
- Krause M., Rodrigues-Krause J., O'Hagan C., Medlow P., Davison G., Susta D., Boreham C., Newsholme P., O'Donnell M., Murphy C., De Vito G. The effects of aerobic exercise training at two different intensities in obesity and type 2 diabetes: implications for oxidative stress, low-grade inflammation and nitric oxide production. Eur. J. Appl. Physiol. 2014;114(2):251–260. doi: 10.1007/s00421-013-2769-6. [DOI] [PubMed] [Google Scholar]
- Krogh-Madsen R., Thyfault J.P., Broholm C., Mortensen O.H., Olsen R.H., Mounier R., Plomgaard P., van Hall G., Booth F.W., Pedersen B.K. A 2-wk reduction of ambulatory activity attenuates peripheral insulin sensitivity. J. Appl. Physiol. 2010;108(5):1034–1040. doi: 10.1152/japplphysiol.00977.2009. [DOI] [PubMed] [Google Scholar]
- Lambernd S., Taube A., Schober A., Platzbecker B., Görgens S.W., Schlich R., Jeruschke K., Weiss J., Eckardt K., Eckel J. Contractile activity of human skeletal muscle cells prevents insulin resistance by inhibiting pro-inflammatory signalling pathways. Diabetologia. 2012;55(4):1128–1139. doi: 10.1007/s00125-012-2454-z. [DOI] [PubMed] [Google Scholar]
- Lund A.J., Hurst T.L., Tyrrell R.M., Thompson D. Markers of chronic inflammation with short-term changes in physical activity. Med. Sci. Sports Exerc. 2011;43(4):578–583. doi: 10.1249/MSS.0b013e3181f59dc4. [DOI] [PubMed] [Google Scholar]
- Marcell T.J., McAuley K.A., Traustadóttir T., Reaven P.D. Exercise training is not associated with improved levels of C-reactive protein or adiponectin. Metabolism. 2005;54(4):533–541. doi: 10.1016/j.metabol.2004.11.008. [DOI] [PubMed] [Google Scholar]
- Markovitch D., Tyrrell R.M., Thompson D. Acute moderate-intensity exercise in middle-aged men has neither an anti- nor proinflammatory effect. J. Appl. Physiol. 2008;105(1):260–265. doi: 10.1152/japplphysiol.00096.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathur M., Pedersen B.K. Exercise as a mean to control low-grade inflammation. Mediat. Inflamm. 2008;2008:109502. doi: 10.1155/2008/109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeilly A.M., McClean C., Murphy M., McEneny J., Trinick T., Burke G., Duly E., McLaughlin J., Davison G. Exercise training and impaired glucose tolerance in obese humans. J. Sports Sci. 2012;30(8):725–732. doi: 10.1080/02640414.2012.671952. [DOI] [PubMed] [Google Scholar]
- Morris J.N., Hardman A.E. Walking to health. Sports Med. 1997;23:306–332. doi: 10.2165/00007256-199723050-00004. [DOI] [PubMed] [Google Scholar]
- Murtagh E.M., Boreham C.A.G., Nevill A., Davison G., Trinick T., Duly E., Al-Agnaf., Murphy E.M. Acute responses of inflammatory markers of cardiovascular disease risk to a single walking session. J. Phys. Act. Health. 2005;2(3):324–332. [Google Scholar]
- Murtagh E.M., Nichols L., Mohammed M.A., Holder R., Nevill A.M., Murphy M.H. The effect of walking on risk factors for cardiovascular disease: an updated systematic review and meta-analysis of randomised control trials. Prev. Med. 2015;72:34–43. doi: 10.1016/j.ypmed.2014.12.041. [DOI] [PubMed] [Google Scholar]
- Navarro S.L., Brasky T.M., Schwarz Y., Song X., Wang C.Y., Kristal A.R., Kratz M., White E., Lampe J.W. Reliability of serum biomarkers of inflammation from repeated measures in healthy individuals. Cancer Epidemiol. Biomarkers Prev. 2012;21(7):1167–1170. doi: 10.1158/1055-9965.EPI-12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R.K., Horowitz J.F. Acute exercise ameliorates differences in insulin resistance between physically active and sedentary overweight adults. Appl. Physiol. Nutr. Metab. 2014;39(7):811–818. doi: 10.1139/apnm-2013-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuparth M.J., Proença J.B., Santos-Silva A., Coimbra S. The positive effect of moderate walking exercise on chemerin levels in Portuguese patients with type 2 diabetes mellitus. J. Investig. Med. 2014;62(2):350–353. doi: 10.2310/JIM.0000000000000025. [DOI] [PubMed] [Google Scholar]
- Nieman D.C., Henson D.A., Austin M.D., Brown V.A. Immune response to a 30-minute walk. Med. Sci. Sports Exerc. 2005;37(1):57–62. doi: 10.1249/01.mss.0000149808.38194.21. [DOI] [PubMed] [Google Scholar]
- Nimmo M.A., Leggate M., Viana J.L., King J.A. The effect of physical activity on mediators of inflammation. Diabetes Obes. Metab. 2013;15(Suppl. 3):51–60. doi: 10.1111/dom.12156. [DOI] [PubMed] [Google Scholar]
- Nishida Y., Higaki Y., Taguchi N., Hara M., Nakamura K., Nanri H., Imaizumi T., Sakamoto T., Horita M., Shinchi K., Tanaka K. Objectively measured physical activity and inflammatory cytokine levels in middle-aged Japanese people. Prev. Med. 2014;64:81–87. doi: 10.1016/j.ypmed.2014.04.004. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscle as an endocrine organ: focus on muscle-derived interleukin-6. Physiol. Rev. 2008;88(4):1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pedersen B.K., Febbraio M.A. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat. Rev. Endocrinol. 2012;8(8):457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- Petersen A.M., Pedersen B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005;98(4):1154–1162. doi: 10.1152/japplphysiol.00164.2004. [DOI] [PubMed] [Google Scholar]
- Phillips S.M., Green H.J., Tarnopolsky M.A., Heigenhauser G.F., Hill R.E., Grant S.M. Effects of training duration on substrate turnover and oxidation during exercise. J. Appl. Physiol. 1996;81(5):2182–2191. doi: 10.1152/jappl.1996.81.5.2182. [DOI] [PubMed] [Google Scholar]
- Pickup J.C. Inflammation and activated innate immunity in the pathogenesis of type 2 diabetes. Diabetes Care. 2004;27(3):813–823. doi: 10.2337/diacare.27.3.813. [DOI] [PubMed] [Google Scholar]
- Pradhan A.D., Manson J.E., Rifai N., Buring J.E., Ridker P.M. C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA. 2001;286(3):327–334. doi: 10.1001/jama.286.3.327. [DOI] [PubMed] [Google Scholar]
- Prince S.A., Adamo K.B., Hamel M.E., Hardt J., Connor Gorber S., Tremblay M. A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review. Int. J. Behav. Nutr. Phys. Act. 2008;5:56. doi: 10.1186/1479-5868-5-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puglisi M.J., Vaishnav U., Shrestha S., Torres-Gonzalez M., Wood R.J., Volek J.S., Fernandez M.L. Raisins and additional walking have distinct effects on plasma lipids and inflammatory cytokines. Lipids Health Dis. 2008;7:14. doi: 10.1186/1476-511X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Cai X., Schumann U., Velders M., Sun Z., Steinacker J.M. Impact of walking on glycemic control and other cardiovascular risk factors in type 2 diabetes: a meta-analysis. PLoS One. 2014;9(10):e109767. doi: 10.1371/journal.pone.0109767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesco E., Tessier S., Lacaille M., Pérusse F., Côté M., Després J.P., Bergeron J., Weisnagel J.S., Doré J., Mauriège P. Impact of a moderate-intensity walking program on cardiometabolic risk markers in overweight to obese women: is there any influence of menopause? Menopause. 2013;20(2):185–193. doi: 10.1097/gme.0b013e31826f7ebf. [DOI] [PubMed] [Google Scholar]
- Schmidt M.I., Duncan B.B., Sharrett A.R., Lindberg G., Savage P.J., Offenbacher S., Azambuja M.I., Tracy R.P., Heiss G. Markers of inflammation and prediction of diabetes mellitus in adults (atherosclerosis risk in communities study): a cohort study. Lancet. 1999;353(9165):1649–1652. doi: 10.1016/s0140-6736(99)01046-6. [DOI] [PubMed] [Google Scholar]
- Shoelson S.E., Lee J., Goldfine A.B. Inflammation and insulin resistance. J. Clin. Invest. 2006;116(7):1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D.T., Carr L.J., Dorozynski C., Gomashe C. Internet-delivered lifestyle physical activity intervention: limited inflammation and antioxidant capacity efficacy in overweight adults. J. Appl. Physiol. 2009;106(1):49–56. doi: 10.1152/japplphysiol.90557.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starkie R., Ostrowski S.R., Jauffred S., Febbraio M., Pedersen B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003;17(8):884–886. doi: 10.1096/fj.02-0670fje. [DOI] [PubMed] [Google Scholar]
- Steensberg A., van Hall G., Osada T., Sacchetti M., Saltin B., Pedersen B.K. Production of interleukin-6 in contracting human skeletal muscles can account for the exercise-induced increase in plasma interleukin-6. J. Physiol. 2000;529(1):237–242. doi: 10.1111/j.1469-7793.2000.00237.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taghian F., Rahnama N., Esfarjani F., Sharifi G.R. Does aerobic exercise effect on the levels of interlukin-6, TNF-α and plasma CRP in the elderly women? Gazz. Med. Ital. Arch. Sci. Med. 2012;171(6):767–773. [Google Scholar]
- Tobina T., Yoshioka K., Hirata A., Mori S., Kiyonaga A., Tanaka H. Peroxisomal proliferator-activated receptor gamma co-activator-1 alpha gene expression increases above the lactate threshold in human skeletal muscle. J. Sports Med. Phys. Fitness. 2011;51(4):683–688. [PubMed] [Google Scholar]
- Tudor-Locke C., Bassett D.R. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34(1):1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- Wellen K.E., Hotamisligil G.S. Inflammation, stress, and diabetes. J. Clin. Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakeu G., Butcher L., Isa S., Webb R., Roberts A.W., Thomas A.W., Backx K., James P.E., Morris K. Low-intensity exercise enhances expression of markers of alternative activation in circulating leukocytes: roles of PPARγ and Th2 cytokines. Atherosclerosis. 2010;212(2):668–673. doi: 10.1016/j.atherosclerosis.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Yates T., Davies M., Brady E., Webb D., Gorely T., Bull F., Talbot D., Sattar N., Khunti K. Walking and inflammatory markers in individuals screened for type 2 diabetes. Prev. Med. 2008;47(4):417–421. doi: 10.1016/j.ypmed.2008.06.015. [DOI] [PubMed] [Google Scholar]
- Yates T., Davies M.J., Gorely T., Talbot D., Bull F., Sattar N., Khunti K. The effect of increased ambulatory activity on markers of chronic low-grade inflammation: evidence from the PREPARE programme randomized controlled trial. Diabet. Med. 2010;27(11):1256–1263. doi: 10.1111/j.1464-5491.2010.03091.x. [DOI] [PubMed] [Google Scholar]
- Yudkin J.S., Stehouwer C.D., Emeis J.J., Coppack S.W. Creactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler. Thromb. Vasc. Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- Zhang K., Shen X., Wu J., Sakaki K., Saunders T., Rutkowski D.T., Back S.H., Kaufman R.J. Endoplasmic reticulum stress activates cleavage of CREBH to induce a systemic inflammatory response. Cell. 2006;124(3):587–599. doi: 10.1016/j.cell.2005.11.040. [DOI] [PubMed] [Google Scholar]
- Zoppini G., Targher G., Zamboni C., Venturi C., Cacciatori V., Moghetti P., Muggeo M. Effects of moderate-intensity exercise training on plasma biomarkers of inflammation and endothelial dysfunction in older patients with type 2 diabetes. Nutr. Metab. Cardiovasc. Dis. 2006;16(8):543–549. doi: 10.1016/j.numecd.2005.09.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material: specific search terms.