Abstract
We previously demonstrated the Healthy Eating and Active Living for Diabetes (HEALD) intervention was effective for increasing daily steps. Here, we consider the cost-effectiveness of the HEALD intervention implemented in primary care.
HEALD was a pedometer-based program for adults with type-2 diabetes in Alberta, Canada completed between January 2010 and September 2012. The main outcome was the change in pedometer-determined steps/day compared to usual care. We estimated total costs per participant for HEALD, and total costs of health care utilization through linkage with administrative health databases. An incremental cost–effectiveness ratio (ICER) was estimated with regression models for differences in costs and effects between study groups.
The HEALD intervention cost $340 per participant over the 6-month follow-up. The difference in total costs (intervention plus health care utilization) was $102 greater per HEALD participant compared to usual care. The intervention group increased their physical activity by 918 steps/day [95% CI 116, 1666] compared to usual care. The resulting ICER was $111 per 1000 steps/day, less than an estimated cost–effectiveness threshold.
Increasing daily steps through an Exercise Specialist-led group program in primary care may be a cost-effective approach towards improving daily physical activity among adults with type-2 diabetes. Alternative delivery strategies may be considered to improve the affordability of this model for primary care.
Keywords: Diabetes, Cost, Walking, Pedometers, Exercise specialists, Primary care
Highlights
-
•
We examined the cost-effectiveness of a diabetes walking program in primary care.
-
•
HEALD was effective in increasing daily steps, costing $340 per participant.
-
•
Physician services, in- and out-patient admissions and intervention costs were used.
-
•
The ICER was $111/1000 steps/day; below our estimated threshold of $176.
-
•
Policymakers must determine the societal value of an additional 1000 steps/day.
Introduction
Improving modifiable risk factors for the prevention and management of chronic diseases like type-2 diabetes (T2D) can be cost-effective. For example, results from the Diabetes Prevention Program suggest the incremental costs of the intensive lifestyle intervention were largely offset by the costs incurred through medications, blood glucose monitoring and hospitalization (inpatient and outpatient) (DPPRG, 2012). More recently, the highly intensive Look AHEAD lifestyle intervention found fewer hospitalizations, lower medication use and lower overall health care costs over 10 years of follow-up despite not achieving the trial's primary target of reduced cardiovascular related morbidity and mortality among adults with T2D (Espeland et al., 2014).
Indeed, the management of diabetes is complex and expensive and is best supported through an interdisciplinary team-based approach (Canadian Diabetes Association, 2013). Healthy eating and active living are key strategies for diabetes self-management, requiring varying support depending on the stage (i.e., more support at the time of diagnosis). Such self-management support requires substantial resource allocation, largely delivered in primary care settings (Bodenheimer et al., 2002); knowing this however, few primary care-based studies have examined the economics of supporting self-management behaviors, including active living (Matthews et al., 2014). Health care utilization and costs appear to be higher among adults with T2D who do not achieve recommended levels of physical activity (Plotnikoff et al., 2008), suggesting that supporting the achievement of physical activity guidelines may offer financial benefit.
Sufficient evidence exists to suggest that physical activity programs for T2D in primary care can be effective for increasing daily physical activity (Plotnikoff et al., 2013, Vuori et al., 2013). We recently completed the Healthy Eating and Active Living for Diabetes in Primary Care Networks (HEALD) trial where we demonstrated that an exercise specialist-led lifestyle program for adults with recently diagnosed T2D in primary care, was effective for increasing daily steps compared to usual care controls (Johnson et al., 2015), along with a comprehensive evaluation of the intervention (Wozniak et al., 2012). Our objective here was to consider the cost-effectiveness of the HEALD intervention versus usual care in a primary care setting.
Methods
Setting and sample
The complete study and evaluation design have been previously reported (Johnson et al., in press, Johnson et al., 2012, Wozniak et al., 2012). Patients recently diagnosed with T2D were recruited from four Primary Care Networks (PCNs) in Alberta, Canada. All PCNs are stand-alone physician-lead corporations established to support family physicians through allied professionals, including: Chronic Disease Management Nurses, Registered Dieticians, Pharmacists, Health Promotion and Prevention Coordinators and Exercise Specialists.
For recruitment, a patient registry that included information about the patient's medical management was used to identify and recruit study participants through each participating PCN. An endorsement letter from each PCN was mailed to the potential participant by staff located at each PCN (Johnson et al., 2012). Only adults (≥ 18 years) who previously received basic diabetes education and were able to read, understand and converse in English were eligible. Those with significant self-reported cardiovascular history on pre-screening (i.e., fainting/dizziness on physical exertion, angina pain, pacemaker, surgery) and current depressive symptoms were also excluded.
All participants were asked to provide their personal health number (PHN) and consent to obtain healthcare utilization data. All data provided was de-identified. The HEALD study was approved by the Health Research Ethics Board at the University of Alberta; ID: Pro00008427. We enrolled 198 people (102 intervention and 96 control) between January 2010 and September 2012, though, for this cost–effectiveness analysis, 186 consented for linkage with health care administrative data (94 intervention and 92 control).
Intervention and control
Eligible participants were allocated using an interrupted time series design (Majumdar et al., 2004). The intervention group participated in a 24-week pedometer-based walking program led by a trained Exercise Specialist at each PCN. All four Exercise Specialists responsible for implementing HEALD had a four-year university degree in Kinesiology/Human Kinetics/Physical Education and professionally certified by the Canadian Society for Exercise Physiology (CESP, 2013). Two of the four Exercise Specialists were employed by their respective PCN, one was seconded from another provincially funded health organization while the third was a casual PCN employee.
For the first 12-weeks of the intervention two group sessions lasted approximately 1 h where participants were provided a pedometer, a logbook and a theory-based resource manual to support individualized goal setting, monitoring, self-efficacy and social support. Participants set their own daily step goals (i.e., a prescribed daily step goal such as 10,000 steps/day was not a component of the program) (Johnson et al., 2012).
After the first 12 weeks, participants were encouraged to walk faster for 30 min 3 days/week for 12 weeks, and to maintain their daily volume step goal (e.g., 7500 steps/day) from the first phase. Two group sessions took place, giving similar resources, but with the behavioral target for brisk walking using a stopwatch. Participants walked on an indoor walking track at a local recreational facility for ~ 30 min, supervised and coached by the exercise specialist, to support the experiential component during both phases.
Those allocated to the usual care control group received a pedometer at baseline with no additional instructions around its use and received usual care during the same 24-week study follow-up period.
Diabetes-related outcomes
Fasting capillary blood samples were collected at point-of-care to assess hemoglobin A1c (DCA Vantage), lipid profile, and glucose (Cholestech, LDX), and resting heart rate and blood pressure were also collected (BPTru). Anthropometric measurements including weight, height, and waist circumference were completed and body mass index (BMI) was calculated. All measures were collected at baseline, 3 and 6 months.
Resource use and cost estimates
Intervention costs included activity time and training of the Exercise Specialists, administrative support personnel, recreation facilities, supplies, equipment, and PCN overhead. Personnel cost estimates were based on the expected group session time, individual counseling and training. Unit costs, including vacation/holiday pay and benefits, were obtained from collective agreements. Overhead cost was assumed 5%, representing the marginal impact on the PCNs. Other component estimates were based on actual costs.
For health care resource use, only direct costs of physician services, hospital outpatient visits and hospital inpatient admissions were included, since the analysis was conducted from a public payer perspective. Since the analytical horizon was less than one year, discounting was not required. All costs were adjusted to 2011 Canadian prices because participant recruitment and follow-up occurred over three years (2009–2012).
To estimate costs we obtained participant (i.e., HEALD and usual care) specific utilization data for physician services, hospital outpatient clinics (including the emergency department) and hospital inpatient services for six months prior to and six months after the completion of the intervention. The provincial government (Alberta Health) uses a fee-for-service system to pay for physician services, based on a standard schedule. For all physician services included in this study, the 2011 schedule was used (AHCIP, 2010). The cost estimates of hospital services were the product the relative cost weight of each service and $6371, the 2011 Alberta cost per weighted case (CIHI, 2012a). The relative cost weights were based on separate case-based grouping. Outpatient weights were scaled so that the cost per weighted inpatient admission could be applied to both outpatient and inpatient events (CIHI, 2012b). The cost estimates of inpatient and outpatient services include drug costs, however, prescription medication costs purchased from community pharmacies were excluded. The cost of operational overhead was included in the estimates for all services.
Health outcome (effectiveness)
The primary outcome was physical activity determined by pedometers; average daily steps, measured and recorded by participants over three days including one weekend day (Tudor-Locke et al., 2005), and collected at baseline, 3 and 6 months.
Cost effectiveness analysis (CEA)
Incremental cost is the difference in the average cost per participant between study groups and the incremental health effect, here, the difference in the average number of steps per participant between study groups. We used a regression framework to adjust incremental cost and health outcome estimates for baseline covariates. Incremental cost was adjusted for differences in 6-month pre-enrolment health care cost and body mass index (BMI). Incremental steps were adjusted for differences in baseline average steps, BMI, age and sex. The incremental cost–effectiveness ratio (ICER) was expressed in 000s of steps, thus representing the additional cost per 1000 additional steps achieved.
In general, cost-effective interventions: cost less (the incremental cost is negative) and are more effective (the incremental health effect is positive), or cost more and are more effective, but society is willing to pay for the additional cost (Drummond et al., 2005). In the latter scenario, the ICER is less than a threshold level that reflects the amount society is willing to pay for an additional unit of health outcome.
Stochastic analysis of uncertainty
To account for uncertainty due to sampling variation, we used a non-parametric bootstrapping analysis to generate a scatter plot of incremental cost and health effect on the cost–effectiveness plane (Drummond et al., 2005). Based on that generated distribution, a cost–effectiveness acceptability curve (CEAC) was derived, indicating the probability of the intervention being cost-effective at various levels of society's willingness to pay per an additional 1000 steps.
We estimated a cost–effectiveness threshold for the intervention in order to interpret the CEAC, since we know of no established benchmark cost–effectiveness threshold for additional steps/day. Following a recently published method, the threshold was estimated as the ratio of average cost to average health effect using the sample data of the control group (Hyewon and Levine, 2012). This threshold reflects the opportunity cost, relating to the health outcome foregone, by the reallocation of resources required to implement the intervention (Claxton et al., 2013).
Statistical analyses
All statistical analyses were performed using R version 3.0.3 [R Foundation for Statistical Computing, Vienna, Austria]. For analyses based on the original sample the Rcmdr package for R, version 2.0-4 [Fox, Bouchet-Valat; Hamilton ON, Canada] was used. Non-parametric bootstrap analysis was conducted with the Boot package for R [Canty, Ripley; Hamilton ON, Canada]. Filemaker Pro Advanced version 13.0 v3 [Filemaker Inc., Santa Clara, USA] was used for general data management.
Missing data
For the 186 participants, only about 3% of the data was missing, and related almost entirely to the primary outcome (steps). Since the proportion of missing data was so small, the last observation carried forward was used to impute missing observations at 6 months and mean value substituted any baseline observations.
Results
The main results have been previously reported (Johnson et al., 2015). Briefly, at baseline the mean age was 59.5 (SD 8.3) years, A1c 6.8% (SD 1.1), 50% women, BMI 33.6 kg/m2 (SD 6.5), systolic pressure 125.6 mm Hg (SD 16.2) and the average daily steps were 5879 (SD 3130). Daily pedometer determined steps increased for the intervention compared to usual care control at 3-months (1292 [SD 2698] vs. 418 [SD 2458]) and 6-months (1481 [SD 2631] vs. 336 [SD 2712]; adjusted p = 0.002).
Intervention participants used fewer health resources both prior to and after enrolment in the study, although no differences were statistically significant (Table 1). HEALD cost $340 per participant, with nearly 60% related to intervention activities and training of the exercise specialists. With the exception of the intervention cost, intervention participants incurred less cost in all categories (physician, out- and in-patient costs) compared to the controls during the follow-up period. The unadjusted mean difference in total cost (intervention plus health care utilization) between groups was insignificant ($4). However, excluding the intervention cost, average cost per intervention participant would be $336 less than for control patients. HEALD participants also incurred fewer costs in the pre-enrollment period. After adjusting for pre-enrolment cost and BMI, the adjusted incremental cost was $102 (Table 2), although not statistically significant. Higher levels of pre-enrolment cost and BMI were significantly associated with higher follow-up costs.
Table 1.
| Pre-enrolment period |
Follow-up period |
|||||
|---|---|---|---|---|---|---|
| Intervention (n = 94) |
Control (n = 92) |
Difference | Intervention (n = 94) |
Control (n = 92) |
Difference | |
| Resource utilization | ||||||
| Physician visits | 8.1 (6.0) | 10.4 (11.4) | − 2.3 (− 0.36, 4.9) | 7.0 (6.0) | 7.8 (6.6) | − 0.8 (− 1.0, 2.6) |
| Outpatient clinic visits | 2.5 (5.1) | 3.1 (4.4) | − 0.6 (− 0.8, 1.9) | 1.4 (2.6) | 2.3 (4.1) | − 0.9 (− 0.1, 1.9) |
| Inpatient admissions | 0.02 (0.15) | 0.08 (0.27) | − 0.06 (− 0.01, 0.12) | 0.01 (0.10) | 0.02 (0.15) | − 0.01 (− 0.03, 0.04) |
| Costc | ||||||
| Intervention | 340 (0) | 0 (0) | 340 (0) | |||
| Physician | 586 (549) | 784 (1043) | − 198 (− 44, 440) | 469 (526) | 573 (613) | − 104 (− 62, 269) |
| Outpatient | 494 (910) | 706 (1230) | − 212 (− 102, 525) | 330 (592) | 477 (761) | − 147 (− 51, 345) |
| Inpatient | 96 (751) | 401 (1605) | − 305 (− 60, 670) | 37 (340) | 122 (913) | − 85 (− 117, 287) |
| Total | 1176 (1619) | 1891 (3240) | − 715 (− 31, 1460) | 1176 (1248) | 1172 (1844) | 4 (− 461, 453) |
Data were collected between January 2010 and September 2012 in Alberta, Canada.
All values within study groups are expressed as average per participant (sd) based on the original sample.
For differences between study groups 95% confidence intervals are shown in parentheses.
2011 Canadian dollars.
Table 2.
Results for cost and health outcome regressionsa.
| Independent variables | Coefficient estimates (t-statistics) |
|
|---|---|---|
| Average cost | Average steps | |
| Study group (1/0) | 102 (0.47) | 919† (2.37) |
| Pre-enrolment cost | 0.21§ (5.06) | |
| Body mass index (BMI) | 33.7† (2.00) | − 76.2† (− 2.51) |
| Baseline average steps | 0.69§ (10.9) | |
| Age | − 71.2‡ (− 2.99) | |
| Male (1/0) | 1093‡ (2.84) | |
| Intercept | − 334 (− 0.59) | 8605§ (4.01) |
| R2 adjusted | 0.13 | 0.51 |
Data were collected between January 2010 and September 2012 in Alberta, Canada.
Based on the original sample.
p-Value < .05.
p-Value < .01.
p-Value < .001.
In terms of health outcome, intervention participants were more active at 6 months, by an adjusted incremental rate of 919 steps, compared with an unadjusted increment of 393 steps (Table 3). In both the original sample and the bootstrapped analysis the incremental health effect was statistically significant (Table 2, Table 3).
Table 3.
Cost–effectiveness analysis.
| Intervention (n = 94) |
Control (n = 92) |
Difference | |
|---|---|---|---|
| Cost per participanta | 1176 | 1172 | 102b (− 318, 464)c |
| Average steps per participant | 7038 | 6645 | 919d (116, 1666)c |
| ICERe | 111 |
Data were collected between January 2010 and September 2012 in Alberta, Canada.
2011 Canadian dollars.
Adjusted for pre-enrolment cost and body mass index (BMI), see Table 2.
Non-parametric bootstrap 95% confidence interval based on 10,000 replications.
Adjusted for baseline average steps, body mass index (BMI), age and sex, see Table 2.
ICER = incremental cost–effectiveness ratio = additional cost per 1000 steps = incremental cost (102) ∕ incremental outcome (919/1000).
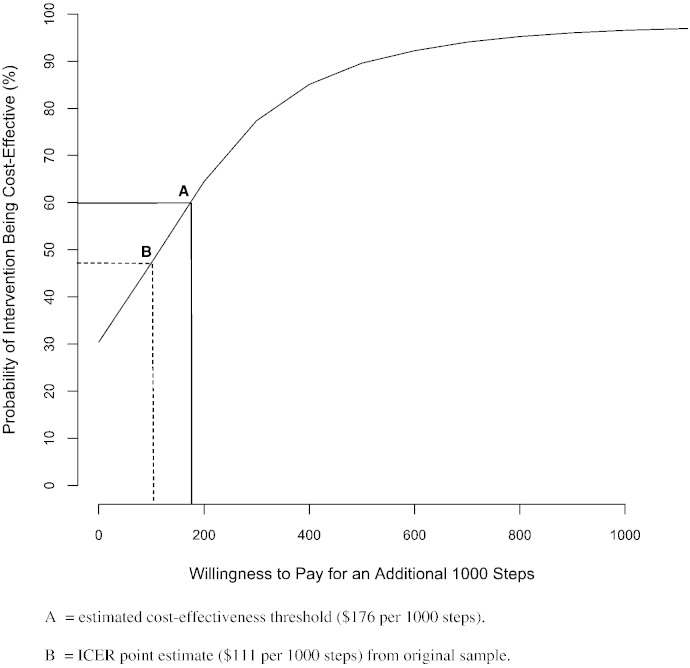
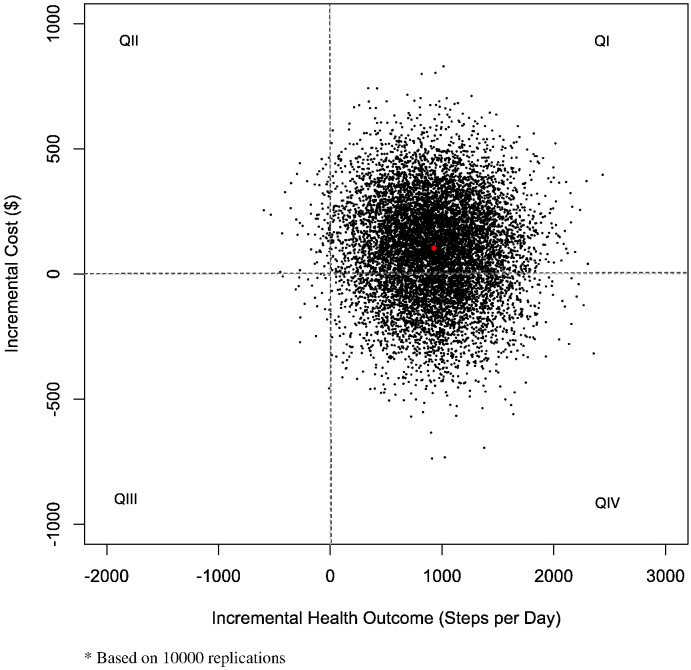
Based on the adjusted incremental estimates, the ICER was $111 per 1000 daily steps (Table 3). The joint distribution of the incremental cost and health effect is shown in Fig. 2, with 69% of the bootstrap replicates located in quadrant I (greater cost, better health outcome) and 30% in quadrant IV, consistent with dominance by the intervention (lower cost, better health outcome). The CEAC (Fig. 1) shows that if society is willing to pay a greater amount for additional steps, the likelihood that the intervention is cost-effective rises steadily to about 97% when willingness-to-pay (WTP) reaches $1000. Based on control group results, we estimated the implied cost-effective threshold to be $176 per additional 1000 steps (point A). At that level, the probability that intervention would be cost-effective is about 60%. The point estimate of the ICER is $111 (point B), clearly less than the estimated threshold.
Fig. 2.
Cost–effectiveness acceptability curve.
Fig. 1.
Bootstrap results on the cost–effectiveness plane*.
*Based on 10,000 replications.
Discussion
Our analysis suggests HEALD may be a cost-effective approach to increasing physical activity in adults with T2D. We conservatively estimated the total intervention cost to be $340 per participant over the 6-month follow-up period. Including intervention costs and health care expenses, the difference in total costs between groups was $102 per participant. Intervention participants increased their physical activity by almost 1000 steps per day, at an incremental cost of $111 compared with usual care.
A common alternative outcome used in economic evaluations is the quality adjusted life years (QALYs) (Shaw et al., 2011). To our knowledge, only one study examined the cost-effectiveness of a lifestyle program in a Canadian T2D population. Using data from the DARE trial (Sigal et al., 2007), Coyle et al. (2012) found that the incremental cost per QALY gained was lowest for a combined aerobic and resistance exercise program against all comparators (i.e., no program, aerobic only and resistance only), concluding that a combined exercise program was most cost-effective for adults with T2D. Our results did not consider QALYs, but rather objectively assessed physical activity behavior (pedometer determined steps), and compared the total costs of both groups.
Given that walking and physical activity are associated with many health benefits (Vuori et al., 2013), QALYs restrict the broad assessment of intervention impacts (Shaw et al., 2011), limiting the true value of increasing physical activity. Neuman, Cohen and Weinstein (2014) comment that theoretically QALYs provide an objective threshold, however, in reality, different approaches provide different values, each based on different assumptions, inferences and contexts. It is impossible to find a single threshold to truly compare interventions (Neuman et al., 2014). In examining cost per daily steps, we provide a unique perspective that has not been previously presented in any chronic disease population. As a novel approach, there is no accepted benchmark or threshold, and it is therefore up to the policymaker to determine the societal value of our outcome, appropriate to the setting and population.
Physical activity interventions can be cost-effective. In their systematic review, Wu et al. (2011) reported comparable cost effectiveness outcomes across a broad range of physical activity interventions by calculating MET-hour gained per day per individual reached. Unlike QALYs, Wu et al., state that this approach accounts for the major parameters of physical activity, including frequency, duration and intensity. In terms of program delivery, they reported that individualized behavior change and social support programs, much like HEALD, were the least cost-effective interventions for increasing daily physical activity. However, they reported that these programs demonstrated greater effect sizes, adding 35%–43% of the guideline-recommended physical activity for adults (Wu et al., 2011). Because they are not only effective, but also costly, these types of programs might be best suited for targeted groups requiring additional support in self-management, like people with T2D.
A recent meta-analysis of randomized controlled trials using pedometers in people with T2D found an increase in daily steps of 1822 across 7 studies (861 participants; 95% confidence interval (CI): 751 to 2894 steps/day) but no improvements in glycemic control (Qiu et al., 2014). Some have suggested that an increase in steps/day of 4000 baseline may be a clinically relevant threshold for improving glycemic control in T2D (Van Dyck et al., 2013). Observational data from the NAVIGATOR trial and AusDIAB study suggests that increases in steps/day above baseline between 1000–2500 reduce the risk of dysglycemia and other cardiovascular related events over 5 years of follow-up among those with a higher cardiometabolic risk profile (Dwyer et al., 2011, Yates et al., 2014). Hence, we argue that although a 1000 steps/day increase might seem low, the increase in daily steps reported here may have behavioral, clinical, and economic relevance.
Our results are important for questions around program implementation and sustainability. It is true that ‘pedometers cost buttons’ (Shaw et al., 2011), but good quality pedometers alone are insufficient to support increases in daily ambulatory activity over the longer term. Pedometers have been likened to a computer without an operating system; that is, providing a pedometer without structured support that incorporates behavioral theory (i.e., goal setting and tracking with a degree of social support) renders the device insufficient for behavior change (Tudor-Locke et al., 2005). HEALD was designed to include behavioral theory and experiential learning in a hospitable environment (a recreational facility with a walking track) and the control group received a pedometer without instruction or support. Hence, the costs beyond simply providing patients with pedometers must be considered by primary care providers to successfully implement a program to increase daily physical activity.
Moreover, HEALD was designed using group sessions, helping to reduce the costs per patient that incur with face-to-face counseling (Vuori et al., 2013). Once well established, more people per session could register, therefore reducing per participant costs. More research is needed to determine the optimal class size for effective and efficient delivery. As well, training can be considered an initial start-up cost, reducing overall cost over time. Regarding program sustainability, by choosing an everyday outcome measure (i.e. steps), continuous program and individual evaluation is possible with minimal resources, extra training and other costs and thus potentially contributing towards long-term program adoption (Matthews et al., 2014).
This analysis has various strengths. The lack of current literature regarding economic evaluation of physical activity interventions and especially controlled trials, attests to the need of this type of analysis. We were able to capture the health care costs of both groups. As well, over 93% of the original participants consented for administrative data linking, maintaining our intended sample size and controlling any differences between the groups of consenting participants. Despite the strengths we have outlined we acknowledge that this study has some weaknesses. Firstly, we used steps/day as our effectiveness measure and because steps are only one component of total daily physical activity the use of an accelerometer may have provided a better objective estimate of daily physical activity across both groups. Nevertheless, steps were the intended target of the intervention. Secondly, we limited our follow-up to a one-year time horizon and hence we have constrained the potential to estimate the long-term impact of the HEALD intervention. Lastly, economic analyses drawn from a healthcare setting where all patients have universal healthcare coverage may limit the generalizability of our findings. For example, one exercise specialist to manage hundreds if not thousands of diabetes patients in this type of setting requires consideration given that they may have other responsibilities (e.g., other patient populations).
In a time of priority shifting and results-based budgeting, without using QALYS, it is difficult to compare the cost-effectiveness of HEALD to other health interventions. Though our analysis is unique, it will take extra effort to present findings to policymakers and reinforce the longer-term impacts and overall health benefits of this walking program. This approach also considers the limitation of the short-term nature of our study. While we considered the cost of hospital, outpatient and physician services, we were unable to estimate differences in costs of lab tests or medications between groups, although given the short-term follow-up, and lack of change in clinical parameters (Johnson et al., 2015), it is unlikely that these differed between study groups.
Conclusion
Health Eating and Active Living for Diabetes (HEALD) was an effective program in increasing daily steps among people with type-2 diabetes in primary care, which over a lifetime, may have worthwhile health benefits. Our economic analysis suggests that the intervention may be cost-effective.
Conflicts and contributions
The authors declare that there are no conflicts of interests. STJ completed the study design and drafted the original manuscript; DL completed the statistical analysis and critically reviewed the manuscript; CM coordinated the study including data collection and management and critically reviewed the manuscript; AS contributed to study coordination and critically reviewed the manuscript; and JAJ contributed to the study design and interpretation of the results along with critical revisions to the manuscript.
All authors had full access to the data and took responsibility for the integrity of the data and the accuracy of the results.
Acknowledgments
This study was funded by through Alberta Health, The Lawson Foundation of Canada (reference #: TLF-CRT 2010-02) and a Canadian Institutes of Health Research (CIHR) Team Grant to the Alliance for Canadian Health Outcomes Research in Diabetes (reference #: OTG-88588), sponsored by the CIHR Institute of Nutrition, Metabolism and Diabetes (INMD). JAJ is an Alberta Innovates Health Solutions Senior Scholar and a Centennial Professor at the University of Alberta.
References
- Alberta Health Care Insurance Plan (AHCIP) 2010. Schedule of Medical Benefits (Procedures and Price List) as of October 1, 2010. Edmonton, Alberta, Canada. [Google Scholar]
- Bodenheimer T., Lorig K., Holman H., Grumbach K. Patient self-management of chronic disease in primary care. JAMA. 2002;288(19):2469–2475. doi: 10.1001/jama.288.19.2469. [DOI] [PubMed] [Google Scholar]
- Canadian Institute for Health Information (CIHI) Hospital financial performance (HFP) indicators. 2012. http://www.cihi.ca Available from: (December 12 2012)
- Canadian Institute for Health Information (CIHI) 2012. CACS Methodology Year 2012. Toronto, Ontario, Canada. [Google Scholar]
- Canadian Diabetes Association Clinical Practice Guidelines Expert Committee Canadian Diabetes Association 2013 Clinical Practice Guidelines for the Prevention and Management of Diabetes in Canada. Can. J. Diab. 2013;37(1):S1–S212. doi: 10.1016/j.jcjd.2013.01.009. [DOI] [PubMed] [Google Scholar]
- Canadian Society for Exercise Physiology CSEP/SCPE: The Gold Standard in Exercise Science and Personal Training. 2013. http://csep.ca/english/view.asp?x=1 Available at: (Accessed December 11, 2013)
- Claxton K., Martin S., Soares M., Rice N., Spackman E., Hinde S., Devlin N., Smith P.C., Sculpher M. Centre for Health Economics, University of York; Heslington, York, UK: 2013. Methods for the Estimation of the NICE Cost Effectiveness Threshold. [Google Scholar]
- Coyle D., Coyle K., Kenny G.P., Boule N.G., Wells G.A., Fortier M., Philips P., Sigal R.J. Cost-effectiveness of exercise programs in type 2 diabetes. Int. J. Technol. Assess. Health Care. 2012;28(3):228–234. doi: 10.1017/S0266462312000256. [DOI] [PubMed] [Google Scholar]
- Diabetes Prevention Program Research Group (DPPRG) The 10-year cost-effectiveness of lifestyle intervention or metformin for diabetes prevention: an intent-to-treat analysis of the DPP/DPPOS. Diabetes Care. 2012;35:723–730. doi: 10.2337/dc11-1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond M.F., Sculper M.J., Torrance G.W., O'Brien B.J., Stoddart G.L. 3rd edn. Oxford Medical Publications, Oxford University Press; New York: 2005. Methods for the Economic Evaluation of Health Care Programmes. [Google Scholar]
- Dwyer T., Ponsonby A.L., Ukoumunne O.C., Pezic A., Venn A., Dunstan D., Barr E., Blair S., Cochrane J., Zimmet P., Shaw J. Association of change in daily step count over five years with insulin sensitivity and adiposity: population based cohort study. Br. Med. J. 2011;342:7249–7252. doi: 10.1136/bmj.c7249. [DOI] [PubMed] [Google Scholar]
- Espeland M.A., Glick H.A., Bertoni A., Look AHEAD Research Group Impact of an intensive lifestyle intervention on use and cost of medical services among overweight and obese adults with type 2 diabetes: the Action for Health in Diabetes. Diabetes Care. 2014;37:2548–2556. doi: 10.2337/dc14-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyewon H.L., Levine M. Determining the threshold of acceptability of an ICER when natural health units are used. J. Popul. Ther. Pharmacol. 2012;19(2):234–238. [PubMed] [Google Scholar]
- Johnson S.T., Mundt C., Soprovich A., Wozniak L., Plotnikoff R.C., Johnson J.A. Healthy eating and active living for diabetes in primary care networks (HEALD-PCN): rationale, design, and evaluation of a pragmatic controlled trial for adults with type 2 diabetes. BMC Public Health. 2012;12:455. doi: 10.1186/1471-2458-12-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson S.T., Mundt C., Qiu W., Soprovich A., Wozniak L., Plotnikoff R.C., Johnson J.A. Increase in daily steps after an exercise specialist led lifestyle intervention for adults with type 2 diabetes in primary care: a controlled implementation trial. J. Phys. Act. Health. 2015 doi: 10.1123/jpah.2014-0200. [DOI] [PubMed] [Google Scholar]
- Majumdar S.R., Rowe B.H., Folk D., Johnson J.A., Holroyd B.H., Morrish D.W., Maksymowych W.P., Steiner I.P., Harley C.H., Wirzba B.J., Hanley D.A., Blitz S., Russell A.S. A controlled trial to increase detection and treatment of osteoporosis in older patients with a wrist fracture. Ann. Intern. Med. 2004;14(5):366–373. doi: 10.7326/0003-4819-141-5-200409070-00011. [DOI] [PubMed] [Google Scholar]
- Matthews L., Kirk A., MacMilan F., Mutrie N. Can physical activity intervention for adults with type 2 diabetes be translated into practice settings? A systematic review using the RE-AIM framework. TBM. 2014;4:60–78. doi: 10.1007/s13142-013-0235-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuman P.J., Cohen J.T., Weinstein M.C. Updating cost-effectiveness — the curious resilience of the $50,000-per QALY threshold. N. Engl. J. Med. 2014;371:796–797. doi: 10.1056/NEJMp1405158. [DOI] [PubMed] [Google Scholar]
- Plotnikoff R.C., Karunamuni N.D., Johnson J.A., Kotovych M., Svenson L.W. Health-related behaviours in adults with diabetes: associations with health care utilization and costs. Can. J. Public Health. 2008;99(3):227–231. doi: 10.1007/BF03405479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plotnikoff R.C., Costigan S.A., Karunamuni N.D., Lubans D.R. Community-based physical activity interventions for treatment of type 2 diabetes: a systematic review with meta-analysis. Front. Endocrinol. 2013;4:3. doi: 10.3389/fendo.2013.00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu S., Cai X., Chen X., Yang B., Sun Z. Step counter use in type 2 diabetes: a meta-analysis of randomized controlled trials. BMC Med. 2014;12:36. doi: 10.1186/1741-7015-12-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw R., Fenwick E., Baker G., McAdam C., Fitzsimmons C., Mutrie N. ‘Pedometers cost buttons’: the feasibility of implementing a pedometer based walking programme within the community. BMC Public Health. 2011;11:200. doi: 10.1186/1471-2458-11-200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigal R.J., Kenny G.P., Boulé N.G., Wells G.A., Prud'homme D., Fortier M., Reid R.D., Tulloch H., Coyle D., Phillips P., Jennings A., Jaffey J. Effects of aerobic training, resistance training, or both on glycemic control in type 2 diabetes: a randomized trial. Ann. Intern. Med. 2007;147(6):357–369. doi: 10.7326/0003-4819-147-6-200709180-00005. [DOI] [PubMed] [Google Scholar]
- Tudor-Locke C., Burkett L., Reis J.P., Ainsworth B.E., Macera C.A., Wilson D.K. How many days of pedometer monitoring predict weekly physical activity in adults? Prev. Med. 2005;40(3):293–298. doi: 10.1016/j.ypmed.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Van Dyck D., De Greef K., Deforche B., Ruige J., Bouckaert J., Tudor-Locke C.E., Kaufman J.M., De Bourdeaudhuij I. The relationship between changes in steps/day and health outcomes after a pedometer-based physical activity intervention with telephone support in type 2 diabetes patients. Health Educ. Res. 2013;28:539–545. doi: 10.1093/her/cyt038. [DOI] [PubMed] [Google Scholar]
- Vuori I.M., Lavie C.J., Blair S.N. Physical activity promotion in the health care system. Mayo Clin. Proc. 2013;88(12):1446–1461. doi: 10.1016/j.mayocp.2013.08.020. [DOI] [PubMed] [Google Scholar]
- Wozniak L., Rees S., Soprovich A., Al Sayah F., Johnson S.T., Majumdar S.R., Johnson J.A. Applying the RE-AIM framework to the Alberta's Caring for Diabetes Project: a protocol for a comprehensive evaluation of primary care quality improvement interventions. BMJ Open. 2012;2:e002099. doi: 10.1136/bmjopen-2012-002099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S., Cohen D., Shi Y., Pearson M., Strum R. Economic analysis of physical activity interventions. Am. J. Prev. Med. 2011;40(2):149–158. doi: 10.1016/j.amepre.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates T., Haffner S.M., Schulte P.J., Thomas L., Huffman K.M., Bales C.W., Califf R.M., Holman R.R., McMurray J.J., Bethel M.A., Tuomilehto J., Davies M.J., Kraus W.E. Association between change in daily ambulatory activity and cardiovascular events in people with impaired glucose tolerance (NAVIGATOR trial): a cohort analysis. Lancet. 2014;383(9922):1059–1066. doi: 10.1016/S0140-6736(13)62061-9. [DOI] [PubMed] [Google Scholar]