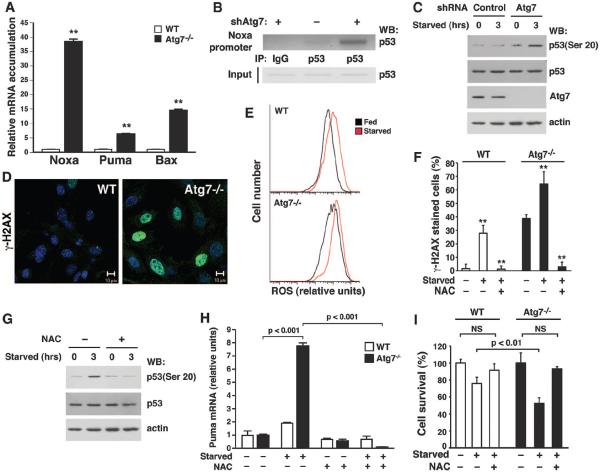
Fig. 3.
The absence of Atg7 increases mitochondrial ROS, augments DNA damage, and activates p53. (A) Relative mRNA expression in starved WT or Atg7−/− MEFs for a panel of proapoptotic p53-responsive genes (**P < 0.01 compared to WT MEFs). (B) ChIP assay 3 hours after the induction of nutrient stress, demonstrating increased p53 binding to the Noxa promoter in HCT116 cells depleted of Atg7. (C) Ser20 phosphorylation in control or Atg7-depleted cells. (D) Images of DNA damage in WT and Atg7−/− MEFs as assessed by γ-H2AX staining. (E) Fluorescence-activated cell sorter analysis of WT and Atg7−/− MEFs under fed (black) or starved (red) conditions, using the redox-dependent fluorophore dichlorodihydrofluorescein diacetate (DCFDA). A rightward shift indicates higher intracellular ROS levels. (F) γ-H2AX foci formation in fed or starved WT and Atg7−/− MEFs. Cells were treated as indicated with the antioxidant NAC (**P < 0.01 compared to starved cells not treated with NAC). (G) NAC treatment inhibits starvation-induced p53 Ser20 phosphorylation in Atg7-depleted cells. (H) Puma expression in WT and Atg7−/− MEFs under fed (−) or starved (+) conditions, in the presence or absence of the cell permeant antioxidant N-acetylcysteine (NAC). (I) Cell death during fed or starved conditions in WT or Atg7−/− MEFs in the presence or absence of NAC. Unless specified, all results are from at least triplicate determinations.