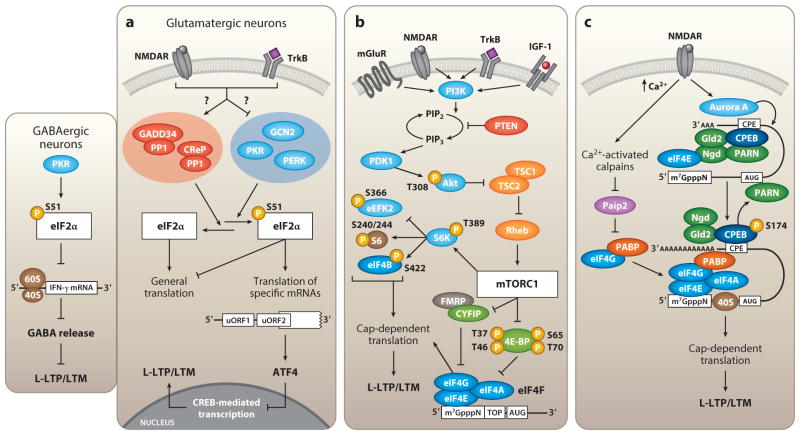
Figure 2.
The key signaling pathways that regulate activity-dependent translation initiation in neurons. (a) Synaptic activity triggers dephosphorylation of initiation factor eIF2α by either activation of eIF2α-specific phosphatase complexes or inhibition of the eIF2α kinases. Dephosphorylation of eIF2α is both sufficient and necessary to induce L-LTP and LTM formation. In glutamatergic neurons, phosphorylation of eIF2α promotes translation of ATF4, a CREB repressor. PKR-mediated phosphorylation of eIF2α in GABAergic neurons negatively regulates translation of the inflammatory cytokine IFN-γ, thus promoting GABA release and maintaining low network rhythmicity in the absence of significant stimulatory inputs. (b) Cap-dependent translation is mediated through mTORC1-dependent phosphorylation of its primary downstream effectors, the 4E-BPs and S6Ks. mTORC1 activity is driven by excitatory synaptic inputs, in addition to other cellular signals, that engage the PI3K/Akt signaling pathway. (c) Calcium influx following NMDAR activation triggers degradation of Paip2 by calcium-activated calpains, thus releasing PABP to bind eIF4G. The eIF4G-PABP complex then contributes to mRNA circularization by directly bridging the elongated 3′ poly(A) tail to the eIF4F complex at the 5′ cap. Cap-dependent translation is initiated upon subsequent recruitment of the 40S and 60S ribosomal complexes. Abbreviations: GADD34, growth arrest and DNA damage-inducible protein; CReP, constitutive reverter of eIF2α phosphorylation; CREB, cAMP response element-binding protein; PI3K, phosphatidylinositide 3-kinase; PDK1, phosphoinositide-dependent kinase-1.