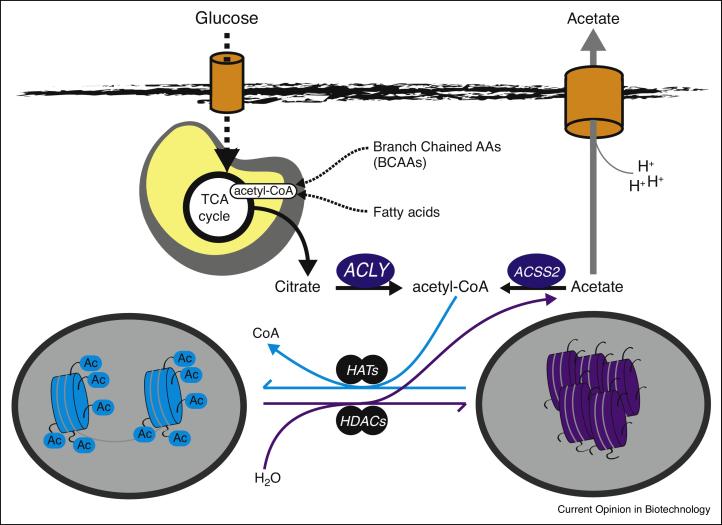
Figure 2.
Acetyl-CoA production and utilization for histone acetylation. ATP-citrate lyase (ACLY) generates nuclear-cytoplasmic acetyl-CoA from nutrients such as glucose, amino acids, and fatty acids. Each of these nutrients generates acetyl-CoA within mitochondria. Transfer of these acetyl units from mitochondria to cytoplasm involves mitochondrial synthesis of citrate from oxaloacetate and acetyl-CoA, then subsequent mitochondrial export and then cleavage of citrate into acetyl-CoA and oxaloacetate by ACLY. Acetyl-CoA serves as the substrate for lysine acetyltransferases (KATs). Histone deacetylases (HDACs) remove acetyl moieties from histones, leading to local production of acetate, which in turn can be recycled back into acetyl-CoA by ACSS2 or exported in a proton-coupled manner to facilitate pH balance. Not shown, the pyruvate dehydrogenase complex has also recently been shown to be present within the nucleus and to provide an additional source of nuclear acetyl-CoA for histone acetylation.