Abstract
Despite advances in surgical techniques, benign biliary strictures after living donor liver transplantation (LDLT) remain a significant biliary complication and play an important role in graft and patient survival. Benign biliary strictures after transplantation are classified into anastomotic or non-anastomotic strictures. These two types differ in presentation, outcome, and response to therapy. The leading causes of biliary strictures include impaired blood supply, technical errors during surgery, and biliary anomalies. Because patients usually have non-specific symptoms, a high index of suspicion should be maintained. Magnetic resonance cholangiography has gained widespread acceptance as a reliable noninvasive tool for detecting biliary complications. Endoscopy has played an increasingly prominent role in the diagnosis and treatment of biliary strictures after LDLT. Endoscopic management in LDLT recipients may be more challenging than in deceased donor liver transplantation patients because of the complex nature of the duct-to-duct reconstruction. Repeated aggressive endoscopic treatment with dilation and the placement of multiple plastic stents is considered the first-line treatment for biliary strictures. Percutaneous and surgical treatments are now reserved for patients for whom endoscopic management fails and for those with multiple, inaccessible intrahepatic strictures or Roux-en-Y anastomoses. Recent advances in enteroscopy enable treatment, even in these latter cases. Direct cholangioscopy, another advanced form of endoscopy, allows direct visualization of the inner wall of the biliary tree and is expected to facilitate stenting or stone extraction. Rendezvous techniques can be a good option when the endoscopic approach to the biliary stricture is unfeasible. These developments have resulted in almost all patients being managed by the endoscopic approach.
Keywords: Biliary strictures, Living donor liver transplantation, Endoscopic retrograde cholangiography, Percutaneous transhepatic cholangiography, Biliary complication
Core tip: The small diameter and complex nature of the duct-to-duct reconstruction of the bile duct in living donor liver transplantation lead to more biliary strictures and difficulties in treatment. A high index of suspicion for the development of biliary stricture should be maintained in order to allow early recognition and early intervention. Nonsurgical methods have become standard therapy. Endoscopic management is generally very effective and has a low incidence of procedure-related complications. Technological advances with newer endoscopic techniques or instruments have continued and may offer the opportunity to widen the indication of endoscopic treatment and to manage more efficiently.
INTRODUCTION
Living transplantation is increasingly being performed worldwide for adults with end-stage liver disease, with over 6000 procedures undertaken annually in the United States and 2000 occurring every year in China. However, the waiting list for liver transplantations grows annually because of a shortage of organs. Living donor liver transplantation (LDLT) is one way to overcome this problem. Asia has the most extensive experience with LDLT; 90% and 70% of total liver transplants are LDLT in Japan and South Korea, respectively.
Despite technical applications and the development of various surgical techniques, biliary complications still occur frequently after transplantation[1-3]. In addition, the small diameter of the anastomotic bile duct leads to more biliary complications in recipients of LDLT compared with patients who have undergone deceased donor liver transplantation (DDLT) (up to 37% vs 10%-15%, respectively)[2,4-7]. Biliary complications after LDLT, particularly benign biliary strictures, play an important role in graft and patient survival. Patients with biliary strictures often require frequent admissions and longer periods of treatment, which can lead to the physical, economical, and even emotional suffering of recipients for extended periods of time. Therefore, the early and precise diagnosis and appropriate management of biliary strictures, which continues to improve over time, is mandatory. In this paper, we will review the current diagnostic methods and modalities for the treatment of biliary strictures after LDLT, including an extensive review of the current literature.
BENIGN STRICTURES AFTER LIVING DONOR TRANSPLANTATION
Classification
Biliary strictures are classified according to their location into an anastomotic stricture (AS) or non-anastomotic stricture (NAS). These two types differ in presentation and outcome after treatment. An AS is usually single and is located at the site of the biliary anastomosis (Figure 1). On the other hand, NASs are usually multiple, and they are located at biliary trees other than the anastomotic sites more than 0.5 cm proximal to the anastomosis (Figure 2)[8]. NASs were first described in DDLT in association with hepatic artery thrombosis, where the biliary tree becomes ischemic and eventually necrotic, resulting in a typical cholangiographic picture of biliary strictures, dilatations, and intraductal cast formation[9,10]. NASs are more diffuse and involve the hilum and multiple separate levels of segmental branch ducts[11]. In NASs, biliary sludge can repeatedly accumulate proximal to the strictures, which leads to the formation of casts and a high incidence of cholangitis. Because the clinical outcomes of the two groups are markedly different, the classification is clinically useful[7].
Figure 1.
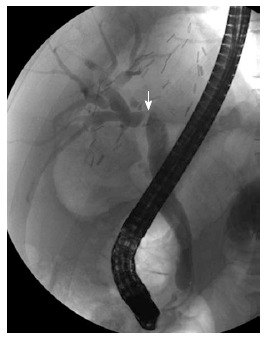
Anastomotic stricture after living donor liver transplantation. Endoscopic retrograde cholangiography shows a single, tight stricture located at the site of the biliary anastomosis (arrow).
Figure 2.
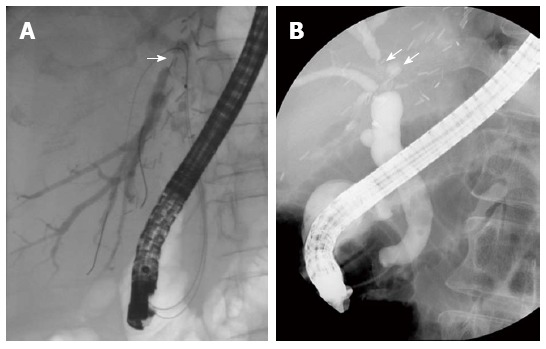
Non-anastomotic strictures after living donor liver transplantation. Endoscopic retrograde cholangiography shows A biliary stricture at distal right posterior sectoral duct above the anastomotic site (A; arrow); A biliary stricture and dilatation at distal right anterior sectoral duct above the anastomotic site (B; arrows).
Incidence and presentation time
The overall incidence of biliary complications in LDLT recipients ranges from 9% to 37%; leaks occur in 5% to 19%, and strictures occur in 4% to 37%[3,12-18]. The incidence of biliary complications after liver transplantation decreased from 30% in the pioneering years to approximately 20% in the 1980s and is currently at 10%[7]. Although the frequency of biliary complications after liver transplantation is gradually decreasing, the rate of bile duct stricture in LDLT remains high, at 10%-37% compared with a rate of 5%-15% in DDLT[18-22]. AS comprises the majority (> 90%) of biliary strictures after LDLT[18,19,21]. NAS in LDLT is less frequent than in DDLT (2%-10% vs 5%-15%, respectively)[10,18,19,21,23,24]. Fortunately, the biliary stricture rates after LDLT have recently declined with center experiences because of improvements in organ selection, the surgical techniques of the biliary reconstruction, and post-operative care. Wang et al[3] reported that the studies published since 2008 have shown a dramatic drop in the overall incidence of biliary complications in LDLT recipients. Another recent study also showed that the decrease in the incidence of biliary strictures occurred during a more recent era (2006-2010), and there was a steady decline in the rate of biliary strictures in transplant patients from 10.9% in 2007 to 3.4% in 2010[25].
Biliary strictures most frequently occur in the early postoperative period. The mean interval for developing a biliary stricture is 5-8 mo after transplantation[14,25]. Within one year, 70%-87% of biliary strictures develop, and the increase in biliary stricture occurrence slows after 1 year[18,25,26]. The incidence of biliary strictures plateaus after approximately 3 years. NAS often presents earlier than AS, with a mean time to stricture development of 3-6 mo[10,27,28].
Causes and risk factors
Biliary complications after LDLT are more frequent than other complications because the bile duct epithelium is more vulnerable to ischemic changes than hepatocyte and vascular epithelia. LDLT is associated with more frequent biliary complications compared with DDLT due to a relatively smaller duct size, leading to a more technically difficult anastomosis and a higher chance of ischemic injury to the allograft[8].
There are many potential causes of biliary strictures, including impaired blood supply (such as hepatic artery thrombosis), technical errors during surgery, biliary anomalies, early bile leaks, organs from elderly donors, longer cold and warm ischemia times, postoperative acute cellular rejection, and long surgical duration[8,26,29]. In multivariate analyses, significant independent risk factors for the development of biliary stricture include donor age greater than 50 years, multiple bile ducts, hepatic artery stenosis, previous history of a bile leak, and a preoperative Model for End-Stage Liver Disease score of greater than or equal to 35[14,17,30,31]. Impaired blood supply causes damage to the bile duct. Because blood supply of the bile duct is mainly from the arterial system[32,33], skeletonization of the duct renders the bile duct ischemic[34]. Hepatic artery thrombosis can lead to complex hilar strictures because blood is supplied to the bile ducts solely via the hepatic artery[8]. Technical issues are considered among the most important etiological factors for AS and include improper surgical technique, such as overly extensive dissection of periductal tissue during procurement or excessive use of electrocautery for biliary duct bleeding control in both the donor and recipient, use of inappropriate suture material, a mismatch in duct size between the donor and recipient bile ducts, small caliber of the bile ducts (< 4 mm), and tension at the anastomosis[7,8,35,36]. Newly developed surgical techniques for preparing bile ducts for biliary anastomosis preserve the maximum blood supply to the bile ducts in both the donors and recipients[12,37-39]. Lin et al[40] reported that the use of microsurgical biliary reconstruction reduced the rate of biliary complications after LDLT to 7% and the rate of complications requiring intervention to 2.5%. The method of bile duct reconstruction and the number of reconstructed bile ducts may also affect the incidence of biliary stricture. Single duct-to-duct anastomosis (DDA) between the right hepatic duct of the graft to the common bile duct of the recipient showed a lower incidence of leakage but a higher incidence of stricture compared with other anastomotic types, including Roux-en-Y choledocho-jejunostomy (CJ) reconstruction[1,20,26,41]. Roux-en-Y is more beneficial than DDA from the viewpoint of arterial collateral formation on the duct stump of the graft[36]. Hwang et al[36] concluded that hepatico-jejunostomy (HJ) is suitable over DDA to reduce AS for right liver grafts, especially those involving ducts less than 4 mm in diameter. However, other studies have reported that there is no definite evidence that methods of bile duct reconstruction are related to AS[16,36], and there were no differences in the incidence of AS in DDA and HJ patients with LDLT[29]. DDA has the advantages of greater physiologic bilioenteric continuity, a lower incidence of leakage, easy endoscopic access to the biliary system, and preservation of the sphincter of Oddi, which plays a role in avoiding reflux of intestinal contents into the bile duct[14,42,43]. Therefore, DDA reconstruction is recognized as a favorable method and a standard technique for adult LDLT. Aberrant biliary anatomy and the presence of 2 or more ducts (which would need multiple biliary reconstructions) are significant risk factors for the development of biliary complications[1,31,44,45]. Kashyap et al[45] reported that the risk of developing biliary complications was 5.9 times higher when the biliary anatomy was any type other than normal. However, in a recent study, there was no association between biliary strictures and the number of ducts[25]. In terms of the use of stents or T-tubes during transplantation, some have reported that bile leaks and cholangitis are higher in patients with T-tubes following DDA, while biliary strictures are higher in non-T-tube recipients[46-50]. However, other studies have shown that there was no significant difference in biliary complications according to the type of biliary stent used in DDA[1,20,51]. A recent meta-analysis revealed that biliary tract reconstruction with a T-tube does not increase the risk of cholangitis, and the T-tube can reduce the risk of biliary stricture in DDLT[52]. This study suggested that biliary reconstruction with a T-tube was still useful and necessary if the recipients had many risk factors for biliary stricture[52].
Although NAS were classified in the early 1990s according to the presence or absence of hepatic artery thrombosis (ischemic or non-ischemic type), the etiology of NAS is multifactorial and heterogeneous[22]. Peribiliary arteriolar endothelial injury results in irreversible microvascular thrombosis and the development of NAS[8]. Early ischemic strictures are associated with hepatic artery compromise, such as thrombosis. The main categories of NAS risk factors include ischemia-related injury, immunologically-induced injury, and cytotoxic injury induced by bile salts[7,10,27,53,54]. Ischemia-related injuries include cold or warm ischemia, hepatic arterial stenosis and thrombosis, donation after cardiac death, reperfusion injury, and injury of the peribiliary vascular plexus[7,10,27]. Immunological injury to the biliary epithelium is related to ABO incompatibility, chronic rejection, pre-existing immunologically mediated diseases, such as primary sclerosing cholangitis and autoimmune hepatitis, and chemokine polymorphisms[10,27]. Less important and inconsistent risk factors include hepatitis C and cytomegalovirus[10,27,53]. However, no specific risk factor can be identified in many NAS cases.
DIAGNOSIS OF BILIARY STRICTURE AFTER LDLT
It is challenging to differentiate biliary strictures as the cause of obstructive jaundice from the many other causes of cholestasis in LT patients, such as acute or chronic rejection, recurrence of the primary disease, fibrosing cholestatic hepatitis C, or medication-related cholestasis[8]. Patients often present with non-specific symptoms or are asymptomatic with laboratory abnormalities, and a diagnosis is therefore usually made on the basis of imaging studies[11].
Clinical features
Patients have various non-specific symptoms, such as anorexia, fever, pruritus, right upper quadrant pain, and/or jaundice. A high index of suspicion should be maintained, as pain is often absent in the transplant recipient because of hepatic denervation and immunosuppression[42,55,56].
Laboratory studies
Studies have shown that biochemical abnormalities, including serum bilirubin, alkaline phosphatase, gamma-glutamyl transferase, and aspartate/alanine aminotransferase, may provide clues to the diagnosis of biliary stricture, even if patients are asymptomatic. However, it is unclear which studies are specific to biliary strictures vs vascular or hepatocellular complications. One study showed that a serum bilirubin > 1.5 mg/dL is a more sensitive indicator of biliary stricture, with 100% sensitivity and 74% specificity[57].
Imaging studies
The characteristic cholangiographic appearance of an AS shows a single, short, localized narrowing in the area of the biliary anastomosis. In NAS, cholangiography shows multiple strictures that are longer in length and proximal to the anastomosis in the extra or intrahepatic bile ducts, resembling primary sclerosing cholangitis[27,28,35].
Initial imaging studies for biliary strictures should include a liver ultrasound (US) with Doppler evaluation of the hepatic vessels to clarify vascular patency[8]. Hepatic angiography is needed when Doppler US indicates hepatic artery stenosis or occlusion. The biliary dilation identified on US serves as a marker of biliary strictures. However, the sensitivity and specificity of abdominal US to detect biliary strictures are not high as the 38%-66% range in liver transplant patients[22]. The presence or absence of biliary dilatation is not a reliable indicator of biliary stricture, particularly in the early postoperative period[58]. In addition, bile duct size is also unreliable for assessing the treatment response. Therefore, when a biliary stricture is strongly suspected, even without positive US findings, a cholangiogram should be performed[6,28,59]. Endoscopic US may have a role in the evaluation of biliary complications after liver transplantation, but it has limitations to evaluate proximal common bile duct and intrahepatic bile duct. Intraductal ultrasonography during endoscopic retrograde cholangiography (ERC) can help to detect or discriminate accompanying biliary stones or casts.
A hepatobiliary iminodiacetic acid scan can represent functional biliary imaging. It is considered excellent for biliary leaks, but its role in detecting biliary strictures is unclear. One study demonstrated that scintigraphy of the hepatobiliary tract using 99 mTc mebrofenin detected biliary strictures with 62% sensitivity and 64% specificity within 30 d, and no patient with normal scintigraphy required biliary intervention[60].
Computed tomography (CT) scanning is useful in evaluating non-biliary lesions and fluid collections due to bile leaks. Multidetector CT has higher spatial resolution compared with magnetic resonance imaging[3]. The sensitivity and specificity of conventional CT to diagnose bile duct strictures is not satisfactory[11]. CT with the intravenous administration of a biliary contrast medium (e.g., iodipamide meglumine) has proven to be efficient in assessing biliary anatomy[61,62], but the risk of allergic reactions with the biliary contrast medium is higher than that with conventional contrast medium. Therefore, its use is limited in many countries.
Magnetic resonance cholangiography (MRC) has gained widespread acceptance as a reliable noninvasive tool for detecting biliary strictures and stones. Recently, MRC has replaced invasive modalities, such as ERC[11]. MRC following US is recommended before ERC or percutaneous treatment if patients carry a higher procedural risk. Because of the inherent high contrast of the bile ducts, MRC can reliably identify most relevant biliary complications, including bile duct strictures[63]. MRC has a high sensitivity (94%-96%) and specificity (94%-95%), as shown in two recent meta-analyses[64,65]. MRC can provide detailed imaging of the entire biliary system both above and below the anastomosis, unlike contrast cholangiography with ERC or percutaneous transhepatic cholangiography (PTC), even if the bile ducts are completely obstructed or disconnected. MRC is also particularly valuable in patients with complex hilar or intrahepatic strictures and an anatomy for which direct cholangiography is difficult to perform[11]. Because MRC provides a detailed road map of the reconstructed bile ducts for interventional procedures or surgery, clinicians may make an optimal treatment plan to reduce morbidity by using MRC. The high negative predictive value of MRC has merit in excluding biliary complications in patients at low-to-moderate risk, thus avoiding unnecessary invasive procedures[63]. There are some disadvantages to MRC, and these include the lack of therapeutic ability, lack of availability, cost-effectiveness for everyday clinical practice, and false positive outcomes for biliary stenosis. MRC often demonstrates some biliary stenosis at the DDA, irrespective of clinical severity or significance, and has a limited ability to detect biliary sludge and small stones (< 5 mm).
Biopsy
Biopsy may suggest the presence of pathology, but it has a limited role[66]. A liver biopsy is often performed to exclude rejection or recurrent fibrosing cholestatic hepatitis C. In LDLT patients with biliary dilation or the presence of common bile duct stones, ERC is initially performed because of the risk of causing a bile leak, and a liver biopsy is warranted if there is no resolution of the cholestasis[8].
TREATMENT OF BILIARY STRICTURE AFTER LDLT
Over the past two decades, the management of biliary strictures has changed from surgical to endoscopic treatment. Unlike an AS, an NAS is more difficult to treat and shows less favorable outcomes, including increased graft loss and death[7]. A team approach, including hepatologists, endoscopists, transplant surgeons, and interventional radiologists, results in the most effective and efficient treatment approach for these patients[8].
Endoscopic retrograde cholangiography
ERC is the mainstay of treatment. Endoscopic management in LDLT recipients may be quite difficult because of the complex nature of duct-to-duct reconstruction[8]. ERC has the advantage over PTC as it allows for the placement of multiple large-caliber stents, and it is more physiological and less invasive. In general, ERC is considered the first therapeutic treatment in patients with DDA.
Endoscopic treatment for biliary strictures includes biliary sphincterotomy, balloon dilation of the stricture, and stent placement (Figure 3)[3]. It had long been debated whether balloon dilation alone or balloon dilation with stent placement is superior for the treatment of biliary strictures. Although stent placement can be associated with a higher complication rate[67], many studies have demonstrated the superiority of balloon dilation with stent placement compared with balloon dilation alone[67-69]. This leads to a general consensus that balloon dilation with biliary stent placement is more effective and has durable outcomes[68,69].
Figure 3.
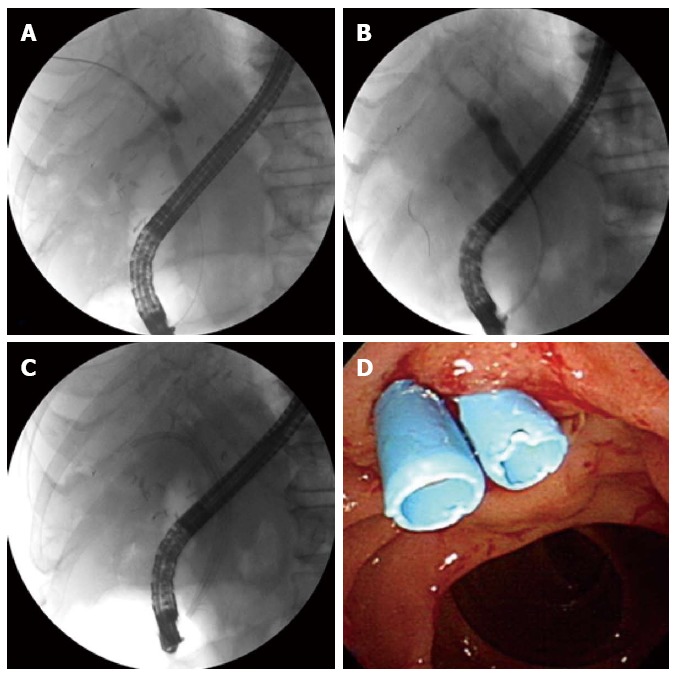
Endoscopic treatment for anastomotic stricture. Endoscopic retrograde cholangiogram shows an anastomotic stricture (A); balloon dilation of the stricture (B); Two 7 Fr plastic stents are placed across the stricture (C); Endoscopy shows the distal ends of two plastic stents (D).
Endoscopic treatment in LDLT patients may be more challenging than in DDLT patients. The endoscopic treatment of AS is less successful in LDLT patients (58%-76%) compared with DDLT patients (80%-90%)[3,14,70-72]. This lower success rate for LDLT patients is attributed to small-caliber/multiple/complex anastomoses or twisted biliary structures that likely result from anastomotic fibrosis and hypertrophy of the transplanted liver[3]. When the bile duct above the stricture is not distended enough or the stricture is too tight or too long for stent placement, endoscopic therapy will not be successful[34,73]. According to the morphology of strictures, pouched type strictures are the most difficult type to manage with endoscopy[34]. Patients with transient narrowing of the anastomosis within the first 1-2 mo after liver transplantation, mainly due to postoperative edema and inflammation, may respond by endoscopic treatment without the need for further treatment[6]. However, the failure rate of primary endoscopic therapy may be high in patients with late onset and delayed diagnosis of biliary stricture after LDLT[31].
Most patients with AS after DDLT require multiple endoscopic sessions every 3 mo with balloon dilation of 6-10 mm and the placement of multiple stents of 7 Fr to 11.5 Fr for 12-24 mo to prevent stent occlusion, stone formation, and bacterial cholangitis[56,70,74,75]. Because of the small caliber of the donor duct, patients with LDLT often require more sessions of endoscopic therapy with the placement of multiple and smaller caliber (7 or 8.5 Fr) stents[11]. One study concluded that a more aggressive strategy in DDLT patients using the placement of the maximum possible number of large diameter stents with accelerated biliary dilation every 2 wk yielded a shorter total length of stenting and a higher success rate (87%)[74]. However, this strategy has some limitations in LDLT patients because of complex and small-caliber biliary anastomoses. Tabibian et al[76] described long-term maximal stent therapy with a 94% success rate, in which the stents are exchanged only when signs or symptoms of biliary obstruction are detected. This strategy differs from general practice and needs to be evaluated in LDLT patients. At the time of the ERC, careful study of the donor cholangiograms is also very useful in detecting biliary anomalies[34]. With advanced techniques, some useful instruments for ERC have been introduced, such as steerable ERC cannulas (e.g., SwingTip cannula, Olympus EndoTherapy, Tokyo, Japan) and multiple guidewires (e.g., VisiGlide, Olympus)[11]. When the strictures are too tight to insert a balloon dilator or stent, the Soehendra biliary dilation catheter or Soehendra stent retriever (Wilson-Cook Medical GI Endoscopy, Winston-Salem, NC, United States) is useful to dilate the stricture site[77].
Endoscopic sphincterotomy is usually performed during ERC for the treatment of AS. Yasumi et al[78] suggested that endoscopic treatment without ES may be a more preferable choice in liver transplant patients because multiple, large, internal stents within the choledochus can avoid compression of the pancreatic orifice, and enterobiliary reflux should be avoided as much as possible after removing the stents (as well as during the stent placement). This has a point because transplant patients have increased vulnerability to infections due to immunosuppressive therapy. Kurita et al[79] recently reported that endoscopic stent placement above the intact sphincter of Oddi for biliary strictures after LDLT achieved long-term stent patency and a high remission rate in patients with a biliary stricture. However, endoscopic sphincterotomy has some advantages in dilating the stricture, placing the stents, and/or removing the stones via the sphincterotomied ampulla in repeated endoscopic sessions. There is still a lack of evidence of the increase in cholangitis- or pancreatitis-related sphincterotomy in liver transplant patients.
Endoscopic therapy is also first-line for NAS treatment, but the outcomes are less satisfactory. NAS are more resistant to endoscopic treatment, with a reported success rate of 25%-75%, whereas the success rate of endoscopic procedures for AS is 77%-90%[19,28,70]. One study showed that the median duration of therapy was 185 d for NAS vs 67 d for AS, and 73% of NAS patients were stent-free 22 mo after the first endoscopic treatment compared with 90% of the AS patients[80]. These results were seen in DDLT patients; thus, data in LDLT patients are still needed. Endoscopic therapy of NAS consists of dilation and stenting of multiple hilar and intrahepatic stenosis lesions, with replacement every few months and extraction of the biliary sludge or casts that may accumulate repeatedly[27]. The upstream duct of the hilar and intrahepatic ducts is often narrowed, which may limit the caliber of the biliary stent or the placement of stents. Because NASs tend to locate in multiple small intrahepatic ducts, balloon dilation of all strictures is frequently infeasible[35]. Although it is not general practice, a research has suggested improved efficacy for NAS and less cholangitis with the use of balloon dilation alone without stenting vs balloon dilation with stenting[11]. Typically, 4 to 6 mm balloon dilation is required in endoscopic therapy of NAS, compared with 6 to 8 mm for AS[8]. NAS often require multiple procedures because of a high recurrence rate, rapid stent occlusion, and recurrent cholangitis. Due to unsatisfactory outcomes of ERC, endoscopic treatment appears to play a role as a bridge to liver retransplantation[35].
The major shortcomings of endoscopic treatments as a standard of care in the management of biliary strictures are the need for multiple procedures repeated over extended periods of time and the risk of cholangitis resulting from stent occlusion. The self-expanding metallic stent (SEMS) has a larger diameter and a longer duration of patency. SEMS has been shown to be superior to plastic stents due to longer stent patency and a single required endoscopic session for malignant biliary strictures[81]. There is some experience in the temporary placement of SEMS for biliary stricture or leak to reduce the need for repeated stent exchanges[82-85]. Uncovered SEMS is rarely used because it induces inevitable reactive hyperplasia that can be accompanied by secondary stone formation above the stent, and removing the stent is a challenge[8]. In comparison, fully covered SEMS can almost always be removed endoscopically, as it is not embeded into the surrounding tissue (Figure 4)[8]. Although SEMS previously appeared to be a promising option in the endoscopic management of biliary strictures after liver transplantation, current evidence does not suggest a clear advantage of SEMS use over multiple plastic stents for biliary stricture after liver transplantation[86]. Migration is a major drawback of covered SEMS. A recent meta-analysis of SEMS for AS showed that the overall SEMS migration rate was significant (16%)[86]. Covered SEMS can also cause strictures in the bile duct, usually secondary to the anchoring point in the distal and proximal end[87]. When it is placed above the biliary bifurcation, SEMS may occlude secondary branch ducts, which limits its use[8].
Figure 4.
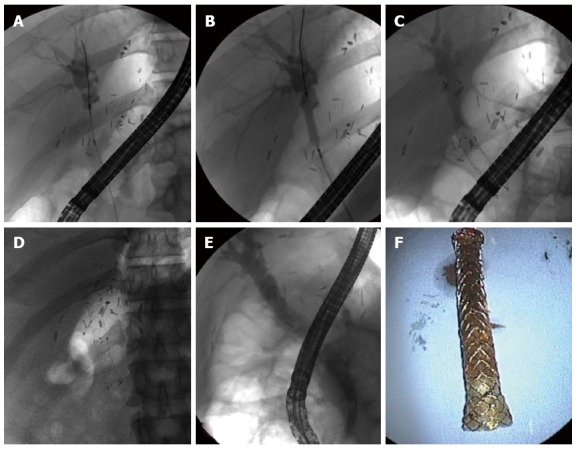
Full-covered self-expanding metallic stent. Endoscopic retrograde cholangiogram shows an anastomotic stricture (A); Balloon dilation of the stricture (B); A metal stent is placed across the stricture (C); The fully dilated metal stent is shown nine months after placement (D); The anastomotic stricture remains in a fully dilated state after removal of the metal stent (E); A removed metal stent (F).
ERC has the limitation of access in the Roux-en-Y reconstruction. In specialized centers, newer endoscopic approaches can be successfully performed to access the biliary strictures using the double balloon enteroscope, single balloon enteroscope, variable stiffness colonoscope, and spiral overtube as an alternative form of PTC for Roux-en-Y patients[15,88-92]. Nonetheless, these relatively new techniques carry some risk of perforation and technical difficulties. These have not yet been established as a standard treatment.
Bile duct stones or biliary casts are also problems frequently seen with biliary strictures during endoscopic therapy. Stones usually form above the bile duct stricture or stenosis, and biliary stents are closely related with stone development. In addition, cyclosporine is known to promote supersaturation of bile and may contribute to the formation of biliary stones[8]. In one series, stones appeared a median interval of 12 mo after liver transplantation, and the stones occurred at the anastomotic site in 90% of stone patients[71]. In our previous report, biliary stones developed in 10.3% of LDLT patients, and recurrent common bile duct or intrahepatic duct stones were found in three of 11 NAS patients[18].
Percutaneous transhepatic cholangiography
PTC is usually reserved for severely strictured or disconnected bile ducts that cannot be traversed by an endoscopic retrograde approach and for patients who have undergone Roux-en-Y reconstruction[15]. Although it is usually successful, percutaneous therapy is regarded as a second-line alternative measure because of its invasive character and its limitation by the size of the percutaneous catheter, which causes the patient discomfort and inconvenience. The other associated complications include bleeding, pseudoaneurysm of the hepatic artery, bile leaks, infection, arterioportal fistula, and portal vein thrombosis[70,93]. If the intrahepatic ducts are not dilated and cannulation under ultrasound guidance is difficult, the risk of vascular injury is higher. The risk of hepatic artery injury with PTC was 2.2% in one study[94]. Gwon et al[95] recently developed a technique using the dual catheter placement technique, namely 2 drainage catheters inserted via a single percutaneous tract. They achieved clinical success in 98.7% of 79 LDLT patients with AS.
Rendezvous technique
The Rendezvous technique is an approach combining PTC and ERC, where access to the biliary tracts is obtained via a percutaneous transhepatic route followed by rendezvous endoscopy[90,96]. When the endoscopic approach to the AS is unobtainable, as in Roux-en-Y reconstructions, this technique can be a good option. As we previously reported, the rendezvous technique is also a safe and useful method for the replacement of the PTBD catheter with the inside stent in patients with an angulated and twisted biliary stricture after LDLT with DDA[97]. The use of the Kumpe catheter as part of the rendezvous technique resulted in a shortened procedure time and an easier operation[58]. The rendezvous technique combining double-balloon endoscopy with percutaneous cholangioscopy has been introduced for the management of biliary stricture after LDLT with Roux-en-Y anastomosis[98].
Surgery
If patients have biliary strictures that are refractory to endoscopic or percutaneous treatment after repeated sessions, surgery should be conducted to prevent septic complications and graft failure[34]. One advantage of surgery is that it eliminates the need for multiple invasive procedures. Surgical management includes the repair of the biliary anastomosis, a conversion from a DD to an HJ anastomosis, and retransplantation[3,28,53,99,100]. Prior PTBD that remains in situ can guide the localization of the bile duct either by fluoroscopy or through the instillation of methylene blue[73]. The long-term surgical results have been good with sustained patient or graft survival[101-103].
PROGNOSIS
The time that an AS is identified is an important factor related to the prognosis. AS occurring less than 6 mo after liver transplantation usually have a better response to treatment and have lower recurrence rates[6]. Pasha et al[104] reported that patients with early onset strictures occurring within 30 d of DDLT required a significantly shorter duration of endoscopic therapy and had a good prognosis[104]. AS occurring more than 6 mo later usually have tight strictures with fibrosis and show high recurrence rates[6,70]. These findings were almost all from DDLT patients with AS. Therefore, further evidence in LDLT patients is still needed. Since biliary strictures after treatment often recur, long-term surveillance with periodic evaluation of liver enzymes and imaging studies is required. Hshei et al[2] reported that recurrent strictures were observed after initial treatment in 21% of LDLT patients, with a median time to recurrence of 9.5 mo, and all recurrences were successfully re-treated endoscopically. Overall, the long-term prognosis in terms of patient and graft survival in patients with AS who are treated appropriately is equivalent to those for matched controls without AS[80,105,106].
The prognosis of NAS is not as favorable as that of AS. NAS often requires significantly longer therapy than AS and carries a risk of secondary biliary cirrhosis. Graft survival is significantly lower in patients with NAS compared with matched controls without NAS[23]. However, the survival of patients with NAS has not changed significantly. The 5-year graft survival of patients with NAS was reported as 50%-70%, and 16% of patients were retransplanted[27,70]. In a prospective study with 749 consecutive patients, the development of NAS significantly attenuated the graft but not patient survival[27]. Because of poor graft survival, early retransplantation is often needed. These results were seen in DDLT patients. The data concerning the prognosis of NAS in LDLT patients are still lacking. After the treatment of NAS, long-term surveillance is also required because NAS usually recur, and complications after treatment are common. Post-treatment cholangitis often develops with sloughing of the biliary epithelium as a result of underlying ischemic or immunologic injury[35].
DONOR-ASSOCIATED BILIARY STRICTURE
In LDLT, donors are also at risk of developing biliary complications. The overall incidence of biliary complications in living liver donors ranges from 0.4% to 13%, and the rates of biliary leaks and strictures range from 0% to 13% and from 0% to 6%, respectively[3,107-113]. Among 393 liver donors in the United States, common complications included biliary leaks (9%), bacterial infections (12%), incisional hernias (6%), and biliary strictures (2%)[111]. The rate of biliary strictures in donors is quite low compared with that in recipients, but it is significant because the donor has been healthy and he/she should recover completely after LDLT. A survey from 42 centers in the United States revealed that the biliary complication (biliary stricture or leak) rate in 449 right liver donors requiring intervention was 6%, reoperation rate was 4.5%, and the death rate was 0.2%[114]. The interventions in liver donors, including ERC, are not different from those of the recipients; however, the bile duct of the liver donor is usually not dilated. Thus, the intervention in liver donors requires more caution.
NEW ATTEMPTS
New types of balloons and stents will play a significant role in improving the management of biliary strictures. Balloon dilation has relatively high primary and secondary failure rates in patients with biliary strictures caused by highly resistant fibrotic changes. New peripheral cutting balloons may be useful in cases of failure of balloon dilation[115]. Paclitaxel-eluting balloons for endotherapy of AS has been introduced[116,117]. The possibility was suggested that paclitaxel-eluting balloons may reduce the need for frequent further invasive interventions. Arain et al[11] introduced the specialized biliary stents to overcome the limitations of stents in NAS. Their stents (Johlin pancreatic wedge stents, Cook Endoscopy) have a large-caliber, are long in length, are of a highly flexible nature, and have multiple side holes. These stents can be placed deep into the intrahepatic ducts, achieve adequate bile drainage through multiple side holes, conform to the tortuous contours according to the left hepatic ducts, and do not migrate.
Advanced endoscopic techniques using direct cholangioscopy (e.g., the SpyGlass Direct Visualization System, Boston Scientific) allow direct visualization of the inner wall of the bile ducts and are currently available in selected centers. Direct cholangioscopy enables the placement of a guidewire across difficult-to-traverse strictures under direct visualization[118,119]. It also may help to differentiate biliary stones from strictures and aid in the directed acquisition of tissues for sampling purposes[11]. Recently, direct per oral cholangioscopy using an ultra-slim (pediatric) forward-viewing video endoscope has been performed in patients with favorable ductal and ampullary anatomy.
Self-expanding stents made of bioabsorbable material has been attempted; this material has theoretical advantages, with longer patency and no need for further interventions. Studies in animal models have shown that bioabsorbable stents offer good patency, no proliferative change, good biocompatibility, and also have a self-clearing effect[120-122]. In addition, bioabsorbable stents can be impregnated with pharmaceutical compounds, such as antimicrobial and antineoplastic agents[77]. In the future, the absorbable biliary stent tube may be clinically developed as a stent for biliary strictures.
Muraoka et al[123] have introduced a magnetic compression anastomosis, which is a novel interventional method that creates an anastomosis between the dilated bile duct and small intestine. The method uses two strong magnets to compress the stricture transmurally, causing gradual ischemic necrosis of the stricture. This ischemic necrosis creates an anastomosis between the two magnets. This technique can be applied to completely obstructed or disconnected biliary strictures[124]. It may be an alternative to open surgery in select cases.
CONCLUSION
During the past 2 decades, there has been a significant decrease in the overall incidence of biliary complications after liver transplantation, including biliary strictures. The understanding of NAS and AS has been widening, and NAS is recognized as one of the most difficult biliary problems to manage. Because patients usually have non-specific symptoms, a high index of suspicion for the development of biliary stricture should be maintained in order to allow early recognition and early endoscopic intervention. MRC is the best non-invasive method to diagnose biliary strictures, and it has high sensitivity and specificity. Nonsurgical methods have become standard therapy for the treatment of biliary strictures in most instances. Endoscopic management is generally very effective and has a low incidence of procedure-related complications. Repeated endoscopic dilation with the placement of multiple plastic stents is the preferred first-line treatment for biliary strictures, avoiding the need for percutaneous transhepatic approaches and surgical management. Technological advances with newer endoscopic techniques or instruments, such as direct cholangioscopy and deep enteroscopy; removable, fully covered metallic biliary stents; and an increasing array of ERC accessory devices, have continued. They may offer the opportunity to widen the indication of endoscopic treatment of post-transplant biliary strictures and to manage them more efficiently in the future. Finally, transplant hepatologists, endoscopists, transplant surgeons, and interventional radiologists should adopt a team approach, which will ultimately result in the best treatment outcomes for biliary strictures after liver transplantation.
ACKNOWLEDGMENTS
Special thanks to our liver transplant center members: Si Hyun Bae, Jong Young Choi, Seung Kyoo Yoon, Young Kyoung You, and Dong Goo Kim.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest to report.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 6, 2015
First decision: August 26, 2015
Article in press: October 13, 2015
P- Reviewer: Boros M S- Editor: Yu J L- Editor: A E- Editor: Zhang DN
References
- 1.Gondolesi GE, Varotti G, Florman SS, Muñoz L, Fishbein TM, Emre SH, Schwartz ME, Miller C. Biliary complications in 96 consecutive right lobe living donor transplant recipients. Transplantation. 2004;77:1842–1848. doi: 10.1097/01.tp.0000123077.78702.0c. [DOI] [PubMed] [Google Scholar]
- 2.Hsieh TH, Mekeel KL, Crowell MD, Nguyen CC, Das A, Aqel BA, Carey EJ, Byrne TJ, Vargas HE, Douglas DD, et al. Endoscopic treatment of anastomotic biliary strictures after living donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2013;77:47–54. doi: 10.1016/j.gie.2012.08.034. [DOI] [PubMed] [Google Scholar]
- 3.Wang SF, Huang ZY, Chen XP. Biliary complications after living donor liver transplantation. Liver Transpl. 2011;17:1127–1136. doi: 10.1002/lt.22381. [DOI] [PubMed] [Google Scholar]
- 4.Trotter JF, Wachs M, Everson GT, Kam I. Adult-to-adult transplantation of the right hepatic lobe from a living donor. N Engl J Med. 2002;346:1074–1082. doi: 10.1056/NEJMra011629. [DOI] [PubMed] [Google Scholar]
- 5.Busuttil RW, Farmer DG, Yersiz H, Hiatt JR, McDiarmid SV, Goldstein LI, Saab S, Han S, Durazo F, Weaver M, et al. Analysis of long-term outcomes of 3200 liver transplantations over two decades: a single-center experience. Ann Surg. 2005;241:905–916; discussion 916-918. doi: 10.1097/01.sla.0000164077.77912.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verdonk RC, Buis CI, Porte RJ, van der Jagt EJ, Limburg AJ, van den Berg AP, Slooff MJ, Peeters PM, de Jong KP, Kleibeuker JH, et al. Anastomotic biliary strictures after liver transplantation: causes and consequences. Liver Transpl. 2006;12:726–735. doi: 10.1002/lt.20714. [DOI] [PubMed] [Google Scholar]
- 7.Koneru B, Sterling MJ, Bahramipour PF. Bile duct strictures after liver transplantation: a changing landscape of the Achilles’ heel. Liver Transpl. 2006;12:702–704. doi: 10.1002/lt.20753. [DOI] [PubMed] [Google Scholar]
- 8.Krok KL, Cárdenas A, Thuluvath PJ. Endoscopic management of biliary complications after liver transplantation. Clin Liver Dis. 2010;14:359–371. doi: 10.1016/j.cld.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 9.Zajko AB, Campbell WL, Logsdon GA, Bron KM, Tzakis A, Esquivel CO, Starzl TE. Cholangiographic findings in hepatic artery occlusion after liver transplantation. AJR Am J Roentgenol. 1987;149:485–489. doi: 10.2214/ajr.149.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buis CI, Hoekstra H, Verdonk RC, Porte RJ. Causes and consequences of ischemic-type biliary lesions after liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:517–524. doi: 10.1007/s00534-005-1080-2. [DOI] [PubMed] [Google Scholar]
- 11.Arain MA, Attam R, Freeman ML. Advances in endoscopic management of biliary tract complications after liver transplantation. Liver Transpl. 2013;19:482–498. doi: 10.1002/lt.23624. [DOI] [PubMed] [Google Scholar]
- 12.Soin AS, Kumaran V, Rastogi AN, Mohanka R, Mehta N, Saigal S, Saraf N, Mohan N, Nundy S. Evolution of a reliable biliary reconstructive technique in 400 consecutive living donor liver transplants. J Am Coll Surg. 2010;211:24–32. doi: 10.1016/j.jamcollsurg.2010.02.048. [DOI] [PubMed] [Google Scholar]
- 13.Lin TS, Concejero AM, Chen CL, Chiang YC, Wang CC, Wang SH, Liu YW, Yang CH, Yong CC, Jawan B, et al. Routine microsurgical biliary reconstruction decreases early anastomotic complications in living donor liver transplantation. Liver Transpl. 2009;15:1766–1775. doi: 10.1002/lt.21947. [DOI] [PubMed] [Google Scholar]
- 14.Shah SA, Grant DR, McGilvray ID, Greig PD, Selzner M, Lilly LB, Girgrah N, Levy GA, Cattral MS. Biliary strictures in 130 consecutive right lobe living donor liver transplant recipients: results of a Western center. Am J Transplant. 2007;7:161–167. doi: 10.1111/j.1600-6143.2006.01601.x. [DOI] [PubMed] [Google Scholar]
- 15.Mita A, Hashikura Y, Masuda Y, Ohno Y, Urata K, Nakazawa Y, Ikegami T, Terada M, Yamamoto H, Miyagawa S. Nonsurgical policy for treatment of bilioenteric anastomotic stricture after living donor liver transplantation. Transpl Int. 2008;21:320–327. doi: 10.1111/j.1432-2277.2007.00609.x. [DOI] [PubMed] [Google Scholar]
- 16.Soejima Y, Taketomi A, Yoshizumi T, Uchiyama H, Harada N, Ijichi H, Yonemura Y, Ikeda T, Shimada M, Maehara Y. Biliary strictures in living donor liver transplantation: incidence, management, and technical evolution. Liver Transpl. 2006;12:979–986. doi: 10.1002/lt.20740. [DOI] [PubMed] [Google Scholar]
- 17.Liu CL, Lo CM, Chan SC, Fan ST. Safety of duct-to-duct biliary reconstruction in right-lobe live-donor liver transplantation without biliary drainage. Transplantation. 2004;77:726–732. doi: 10.1097/01.tp.0000116604.89083.2f. [DOI] [PubMed] [Google Scholar]
- 18.Chang JH, Lee IS, Choi JY, Yoon SK, Kim DG, You YK, Chun HJ, Lee DK, Choi MG, Chung IS. Biliary Stricture after Adult Right-Lobe Living-Donor Liver Transplantation with Duct-to-Duct Anastomosis: Long-Term Outcome and Its Related Factors after Endoscopic Treatment. Gut Liver. 2010;4:226–233. doi: 10.5009/gnl.2010.4.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yazumi S, Yoshimoto T, Hisatsune H, Hasegawa K, Kida M, Tada S, Uenoyama Y, Yamauchi J, Shio S, Kasahara M, et al. Endoscopic treatment of biliary complications after right-lobe living-donor liver transplantation with duct-to-duct biliary anastomosis. J Hepatobiliary Pancreat Surg. 2006;13:502–510. doi: 10.1007/s00534-005-1084-y. [DOI] [PubMed] [Google Scholar]
- 20.Kasahara M, Egawa H, Takada Y, Oike F, Sakamoto S, Kiuchi T, Yazumi S, Shibata T, Tanaka K. Biliary reconstruction in right lobe living-donor liver transplantation: Comparison of different techniques in 321 recipients. Ann Surg. 2006;243:559–566. doi: 10.1097/01.sla.0000206419.65678.2e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tsujino T, Isayama H, Sugawara Y, Sasaki T, Kogure H, Nakai Y, Yamamoto N, Sasahira N, Yamashiki N, Tada M, et al. Endoscopic management of biliary complications after adult living donor liver transplantation. Am J Gastroenterol. 2006;101:2230–2236. doi: 10.1111/j.1572-0241.2006.00797.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma S, Gurakar A, Camci C, Jabbour N. Avoiding pitfalls: what an endoscopist should know in liver transplantation--part II. Dig Dis Sci. 2009;54:1386–1402. doi: 10.1007/s10620-008-0520-7. [DOI] [PubMed] [Google Scholar]
- 23.Verdonk RC, Buis CI, van der Jagt EJ, Gouw AS, Limburg AJ, Slooff MJ, Kleibeuker JH, Porte RJ, Haagsma EB. Nonanastomotic biliary strictures after liver transplantation, part 2: Management, outcome, and risk factors for disease progression. Liver Transpl. 2007;13:725–732. doi: 10.1002/lt.21165. [DOI] [PubMed] [Google Scholar]
- 24.Buis CI, Verdonk RC, Van der Jagt EJ, van der Hilst CS, Slooff MJ, Haagsma EB, Porte RJ. Nonanastomotic biliary strictures after liver transplantation, part 1: Radiological features and risk factors for early vs. late presentation. Liver Transpl. 2007;13:708–718. doi: 10.1002/lt.21166. [DOI] [PubMed] [Google Scholar]
- 25.Kim PT, Marquez M, Jung J, Cavallucci D, Renner EL, Cattral M, Greig PD, McGilvray ID, Selzner M, Ghanekar A, et al. Long-term follow-up of biliary complications after adult right-lobe living donor liver transplantation. Clin Transplant. 2015;29:465–474. doi: 10.1111/ctr.12538. [DOI] [PubMed] [Google Scholar]
- 26.Melcher ML, Pomposelli JJ, Verbesey JE, McTaggart RA, Freise CE, Ascher NL, Roberts JP, Pomfret EA. Comparison of biliary complications in adult living-donor liver transplants performed at two busy transplant centers. Clin Transplant. 2010;24:E137–E144. doi: 10.1111/j.1399-0012.2009.01189.x. [DOI] [PubMed] [Google Scholar]
- 27.Guichelaar MM, Benson JT, Malinchoc M, Krom RA, Wiesner RH, Charlton MR. Risk factors for and clinical course of non-anastomotic biliary strictures after liver transplantation. Am J Transplant. 2003;3:885–890. doi: 10.1034/j.1600-6143.2003.00165.x. [DOI] [PubMed] [Google Scholar]
- 28.Graziadei IW, Schwaighofer H, Koch R, Nachbaur K, Koenigsrainer A, Margreiter R, Vogel W. Long-term outcome of endoscopic treatment of biliary strictures after liver transplantation. Liver Transpl. 2006;12:718–725. doi: 10.1002/lt.20644. [DOI] [PubMed] [Google Scholar]
- 29.Chok KS, Chan SC, Cheung TT, Sharr WW, Chan AC, Lo CM, Fan ST. Bile duct anastomotic stricture after adult-to-adult right lobe living donor liver transplantation. Liver Transpl. 2011;17:47–52. doi: 10.1002/lt.22188. [DOI] [PubMed] [Google Scholar]
- 30.Welling TH, Heidt DG, Englesbe MJ, Magee JC, Sung RS, Campbell DA, Punch JD, Pelletier SJ. Biliary complications following liver transplantation in the model for end-stage liver disease era: effect of donor, recipient, and technical factors. Liver Transpl. 2008;14:73–80. doi: 10.1002/lt.21354. [DOI] [PubMed] [Google Scholar]
- 31.Seo JK, Ryu JK, Lee SH, Park JK, Yang KY, Kim YT, Yoon YB, Lee HW, Yi NJ, Suh KS. Endoscopic treatment for biliary stricture after adult living donor liver transplantation. Liver Transpl. 2009;15:369–380. doi: 10.1002/lt.21700. [DOI] [PubMed] [Google Scholar]
- 32.Stapleton GN, Hickman R, Terblanche J. Blood supply of the right and left hepatic ducts. Br J Surg. 1998;85:202–207. doi: 10.1046/j.1365-2168.1998.00511.x. [DOI] [PubMed] [Google Scholar]
- 33.Couinaud C. The parabiliary venous system. Surg Radiol Anat. 1988;10:311–316. doi: 10.1007/BF02107904. [DOI] [PubMed] [Google Scholar]
- 34.Chok KS, Lo CM. Prevention and management of biliary anastomotic stricture in right-lobe living-donor liver transplantation. J Gastroenterol Hepatol. 2014;29:1756–1763. doi: 10.1111/jgh.12648. [DOI] [PubMed] [Google Scholar]
- 35.Williams ED, Draganov PV. Endoscopic management of biliary strictures after liver transplantation. World J Gastroenterol. 2009;15:3725–3733. doi: 10.3748/wjg.15.3725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hwang S, Lee SG, Sung KB, Park KM, Kim KH, Ahn CS, Lee YJ, Lee SK, Hwang GS, Moon DB, et al. Long-term incidence, risk factors, and management of biliary complications after adult living donor liver transplantation. Liver Transpl. 2006;12:831–838. doi: 10.1002/lt.20693. [DOI] [PubMed] [Google Scholar]
- 37.Marubashi S, Dono K, Nagano H, Kobayashi S, Takeda Y, Umeshita K, Monden M, Doki Y, Mori M. Biliary reconstruction in living donor liver transplantation: technical invention and risk factor analysis for anastomotic stricture. Transplantation. 2009;88:1123–1130. doi: 10.1097/TP.0b013e3181ba184a. [DOI] [PubMed] [Google Scholar]
- 38.Kim SH, Lee KW, Kim YK, Cho SY, Han SS, Park SJ. Tailored telescopic reconstruction of the bile duct in living donor liver transplantation. Liver Transpl. 2010;16:1069–1074. doi: 10.1002/lt.22116. [DOI] [PubMed] [Google Scholar]
- 39.Soejima Y, Fukuhara T, Morita K, Yoshizumi T, Ikegami T, Yamashita Y, Sugimachi K, Taketomi A, Maehara Y. A simple hilar dissection technique preserving maximum blood supply to the bile duct in living donor liver transplantation. Transplantation. 2008;86:1468–1469. doi: 10.1097/TP.0b013e318188d4dc. [DOI] [PubMed] [Google Scholar]
- 40.Lin TS, Chen CL, Concejero AM, Yap AQ, Lin YH, Liu CY, Chiang YC, Wang CC, Wang SH, Lin CC, et al. Early and long-term results of routine microsurgical biliary reconstruction in living donor liver transplantation. Liver Transpl. 2013;19:207–214. doi: 10.1002/lt.23582. [DOI] [PubMed] [Google Scholar]
- 41.Chok KS, Chan SC, Chan KL, Sharr WW, Tam PK, Fan ST, Lo CM. Bile duct anastomotic stricture after pediatric living donor liver transplantation. J Pediatr Surg. 2012;47:1399–1403. doi: 10.1016/j.jpedsurg.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 42.Pascher A, Neuhaus P. Biliary complications after deceased-donor orthotopic liver transplantation. J Hepatobiliary Pancreat Surg. 2006;13:487–496. doi: 10.1007/s00534-005-1083-z. [DOI] [PubMed] [Google Scholar]
- 43.Sung JY, Costerton JW, Shaffer EA. Defense system in the biliary tract against bacterial infection. Dig Dis Sci. 1992;37:689–696. doi: 10.1007/BF01296423. [DOI] [PubMed] [Google Scholar]
- 44.Testa G, Malagó M, Valentín-Gamazo C, Lindell G, Broelsch CE. Biliary anastomosis in living related liver transplantation using the right liver lobe: techniques and complications. Liver Transpl. 2000;6:710–714. doi: 10.1053/jlts.2000.18706. [DOI] [PubMed] [Google Scholar]
- 45.Kashyap R, Bozorgzadeh A, Abt P, Tsoulfas G, Maloo M, Sharma R, Patel S, Dombroski D, Mantry P, Safadjou S, et al. Stratifying risk of biliary complications in adult living donor liver transplantation by magnetic resonance cholangiography. Transplantation. 2008;85:1569–1572. doi: 10.1097/TP.0b013e31816ff21f. [DOI] [PubMed] [Google Scholar]
- 46.Rabkin JM, Orloff SL, Reed MH, Wheeler LJ, Corless CL, Benner KG, Flora KD, Rosen HR, Olyaei AJ. Biliary tract complications of side-to-side without T tube versus end-to-end with or without T tube choledochocholedochostomy in liver transplant recipients. Transplantation. 1998;65:193–199. doi: 10.1097/00007890-199801270-00008. [DOI] [PubMed] [Google Scholar]
- 47.Amador A, Charco R, Marti J, Alvarez G, Ferrer J, Mans E, Fuster J, Fondevila C, Garcia-Valdecasas JC. Cost/efficacy clinical trial about the use of T-tube in cadaveric donor liver transplant: preliminary results. Transplant Proc. 2005;37:1129–1130. doi: 10.1016/j.transproceed.2005.01.015. [DOI] [PubMed] [Google Scholar]
- 48.Scatton O, Meunier B, Cherqui D, Boillot O, Sauvanet A, Boudjema K, Launois B, Fagniez PL, Belghiti J, Wolff P, et al. Randomized trial of choledochocholedochostomy with or without a T tube in orthotopic liver transplantation. Ann Surg. 2001;233:432–437. doi: 10.1097/00000658-200103000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vougas V, Rela M, Gane E, Muiesan P, Melendez HV, Williams R, Heaton ND. A prospective randomised trial of bile duct reconstruction at liver transplantation: T tube or no T tube? Transpl Int. 1996;9:392–395. doi: 10.1007/BF00335701. [DOI] [PubMed] [Google Scholar]
- 50.Sotiropoulos GC, Sgourakis G, Radtke A, Molmenti EP, Goumas K, Mylona S, Fouzas I, Karaliotas C, Lang H. Orthotopic liver transplantation: T-tube or not T-tube? Systematic review and meta-analysis of results. Transplantation. 2009;87:1672–1680. doi: 10.1097/TP.0b013e3181a5cf3f. [DOI] [PubMed] [Google Scholar]
- 51.Akamatsu N, Sugawara Y, Hashimoto D. Biliary reconstruction, its complications and management of biliary complications after adult liver transplantation: a systematic review of the incidence, risk factors and outcome. Transpl Int. 2011;24:379–392. doi: 10.1111/j.1432-2277.2010.01202.x. [DOI] [PubMed] [Google Scholar]
- 52.Sun N, Zhang J, Li X, Zhang C, Zhou X, Zhang C. Biliary tract reconstruction with or without T-tube in orthotopic liver transplantation: a systematic review and meta-analysis. Expert Rev Gastroenterol Hepatol. 2015;9:529–538. doi: 10.1586/17474124.2015.1002084. [DOI] [PubMed] [Google Scholar]
- 53.Colonna JO, Shaked A, Gomes AS, Colquhoun SD, Jurim O, McDiarmid SV, Millis JM, Goldstein LI, Busuttil RW. Biliary strictures complicating liver transplantation. Incidence, pathogenesis, management, and outcome. Ann Surg. 1992;216:344–350; discussion 350-352. doi: 10.1097/00000658-199209000-00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abt P, Crawford M, Desai N, Markmann J, Olthoff K, Shaked A. Liver transplantation from controlled non-heart-beating donors: an increased incidence of biliary complications. Transplantation. 2003;75:1659–1663. doi: 10.1097/01.TP.0000062574.18648.7C. [DOI] [PubMed] [Google Scholar]
- 55.Verdonk RC, Buis CI, Porte RJ, Haagsma EB. Biliary complications after liver transplantation: a review. Scand J Gastroenterol Suppl. 2006;(243):89–101. doi: 10.1080/00365520600664375. [DOI] [PubMed] [Google Scholar]
- 56.Thuluvath PJ, Pfau PR, Kimmey MB, Ginsberg GG. Biliary complications after liver transplantation: the role of endoscopy. Endoscopy. 2005;37:857–863. doi: 10.1055/s-2005-870192. [DOI] [PubMed] [Google Scholar]
- 57.Venu M, Brown RD, Lepe R, Berkes J, Cotler SJ, Benedetti E, Testa G, Venu RP. Laboratory diagnosis and nonoperative management of biliary complications in living donor liver transplant patients. J Clin Gastroenterol. 2007;41:501–506. doi: 10.1097/01.mcg.0000247986.95053.2a. [DOI] [PubMed] [Google Scholar]
- 58.Chang JH, Lee IS, Chun HJ, Choi JY, Yoon SK, Kim DG, You YK, Choi MG, Han SW. Comparative study of rendezvous techniques in post-liver transplant biliary stricture. World J Gastroenterol. 2012;18:5957–5964. doi: 10.3748/wjg.v18.i41.5957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zemel G, Zajko AB, Skolnick ML, Bron KM, Campbell WL. The role of sonography and transhepatic cholangiography in the diagnosis of biliary complications after liver transplantation. AJR Am J Roentgenol. 1988;151:943–946. doi: 10.2214/ajr.151.5.943. [DOI] [PubMed] [Google Scholar]
- 60.Kurzawinski TR, Selves L, Farouk M, Dooley J, Hilson A, Buscombe JR, Burroughs A, Rolles K, Davidson BR. Prospective study of hepatobiliary scintigraphy and endoscopic cholangiography for the detection of early biliary complications after orthotopic liver transplantation. Br J Surg. 1997;84:620–623. [PubMed] [Google Scholar]
- 61.Cheng YF, Lee TY, Chen CL, Huang TL, Chen YS, Lui CC. Three-dimensional helical computed tomographic cholangiography: application to living related hepatic transplantation. Clin Transplant. 1997;11:209–213. [PubMed] [Google Scholar]
- 62.Schroeder T, Malagó M, Debatin JF, Testa G, Nadalin S, Broelsch CE, Ruehm SG. Multidetector computed tomographic cholangiography in the evaluation of potential living liver donors. Transplantation. 2002;73:1972–1973. doi: 10.1097/00007890-200206270-00026. [DOI] [PubMed] [Google Scholar]
- 63.Girometti R, Cereser L, Bazzocchi M, Zuiani C. Magnetic resonance cholangiography in the assessment and management of biliary complications after OLT. World J Radiol. 2014;6:424–436. doi: 10.4329/wjr.v6.i7.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Xu YB, Min ZG, Jiang HX, Qin SY, Hu BL. Diagnostic value of magnetic resonance cholangiopancreatography for biliary complications in orthotopic liver transplantation: a meta-analysis. Transplant Proc. 2013;45:2341–2346. doi: 10.1016/j.transproceed.2013.03.031. [DOI] [PubMed] [Google Scholar]
- 65.Jorgensen JE, Waljee AK, Volk ML, Sonnenday CJ, Elta GH, Al-Hawary MM, Singal AG, Taylor JR, Elmunzer BJ. Is MRCP equivalent to ERCP for diagnosing biliary obstruction in orthotopic liver transplant recipients? A meta-analysis. Gastrointest Endosc. 2011;73:955–962. doi: 10.1016/j.gie.2010.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sebagh M, Yilmaz F, Karam V, Falissard B, Roche B, Azoulay D, Samuel D, Guettier C. The histologic pattern of “biliary tract pathology” is accurate for the diagnosis of biliary complications. Am J Surg Pathol. 2005;29:318–323. doi: 10.1097/01.pas.0000152139.66524.ab. [DOI] [PubMed] [Google Scholar]
- 67.Kulaksiz H, Weiss KH, Gotthardt D, Adler G, Stremmel W, Schaible A, Dogan A, Stiehl A, Sauer P. Is stenting necessary after balloon dilation of post-transplantation biliary strictures? Results of a prospective comparative study. Endoscopy. 2008;40:746–751. doi: 10.1055/s-2008-1077489. [DOI] [PubMed] [Google Scholar]
- 68.Schwartz DA, Petersen BT, Poterucha JJ, Gostout CJ. Endoscopic therapy of anastomotic bile duct strictures occurring after liver transplantation. Gastrointest Endosc. 2000;51:169–174. doi: 10.1016/s0016-5107(00)70413-5. [DOI] [PubMed] [Google Scholar]
- 69.Zoepf T, Maldonado-Lopez EJ, Hilgard P, Malago M, Broelsch CE, Treichel U, Gerken G. Balloon dilatation vs. balloon dilatation plus bile duct endoprostheses for treatment of anastomotic biliary strictures after liver transplantation. Liver Transpl. 2006;12:88–94. doi: 10.1002/lt.20548. [DOI] [PubMed] [Google Scholar]
- 70.Sharma S, Gurakar A, Jabbour N. Biliary strictures following liver transplantation: past, present and preventive strategies. Liver Transpl. 2008;14:759–769. doi: 10.1002/lt.21509. [DOI] [PubMed] [Google Scholar]
- 71.Park JS, Kim MH, Lee SK, Seo DW, Lee SS, Han J, Min YI, Hwang S, Park KM, Lee YJ, et al. Efficacy of endoscopic and percutaneous treatments for biliary complications after cadaveric and living donor liver transplantation. Gastrointest Endosc. 2003;57:78–85. doi: 10.1067/mge.2003.11. [DOI] [PubMed] [Google Scholar]
- 72.Kato H, Kawamoto H, Tsutsumi K, Harada R, Fujii M, Hirao K, Kurihara N, Mizuno O, Ishida E, Ogawa T, et al. Long-term outcomes of endoscopic management for biliary strictures after living donor liver transplantation with duct-to-duct reconstruction. Transpl Int. 2009;22:914–921. doi: 10.1111/j.1432-2277.2009.00895.x. [DOI] [PubMed] [Google Scholar]
- 73.Chan SC, Fan ST. Biliary complications in liver transplantation. Hepatol Int. 2008;2:399–404. doi: 10.1007/s12072-008-9092-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Morelli G, Fazel A, Judah J, Pan JJ, Forsmark C, Draganov P. Rapid-sequence endoscopic management of posttransplant anastomotic biliary strictures. Gastrointest Endosc. 2008;67:879–885. doi: 10.1016/j.gie.2007.08.046. [DOI] [PubMed] [Google Scholar]
- 75.Morelli J, Mulcahy HE, Willner IR, Cunningham JT, Draganov P. Long-term outcomes for patients with post-liver transplant anastomotic biliary strictures treated by endoscopic stent placement. Gastrointest Endosc. 2003;58:374–379. doi: 10.1067/s0016-5107(03)00011-7. [DOI] [PubMed] [Google Scholar]
- 76.Tabibian JH, Asham EH, Han S, Saab S, Tong MJ, Goldstein L, Busuttil RW, Durazo FA. Endoscopic treatment of postorthotopic liver transplantation anastomotic biliary strictures with maximal stent therapy (with video) Gastrointest Endosc. 2010;71:505–512. doi: 10.1016/j.gie.2009.10.023. [DOI] [PubMed] [Google Scholar]
- 77.Ryu CH, Lee SK. Biliary strictures after liver transplantation. Gut Liver. 2011;5:133–142. doi: 10.5009/gnl.2011.5.2.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yazumi S, Chiba T. Endoscopic management of biliary stricture after right-lobe living-donor liver transplantation with biliary anastomosis. Clin Gastroenterol Hepatol. 2006;4:1296; author reply 1296. doi: 10.1016/j.cgh.2006.07.019. [DOI] [PubMed] [Google Scholar]
- 79.Kurita A, Kodama Y, Minami R, Sakuma Y, Kuriyama K, Tanabe W, Ohta Y, Maruno T, Shiokawa M, Sawai Y, et al. Endoscopic stent placement above the intact sphincter of Oddi for biliary strictures after living donor liver transplantation. J Gastroenterol. 2013;48:1097–1104. doi: 10.1007/s00535-012-0705-x. [DOI] [PubMed] [Google Scholar]
- 80.Rizk RS, McVicar JP, Emond MJ, Rohrmann CA, Kowdley KV, Perkins J, Carithers RL, Kimmey MB. Endoscopic management of biliary strictures in liver transplant recipients: effect on patient and graft survival. Gastrointest Endosc. 1998;47:128–135. doi: 10.1016/s0016-5107(98)70344-x. [DOI] [PubMed] [Google Scholar]
- 81.Vitale GC, Larson GM, George M, Tatum C. Management of malignant biliary stricture with self-expanding metallic stent. Surg Endosc. 1996;10:970–973. doi: 10.1007/s004649900216. [DOI] [PubMed] [Google Scholar]
- 82.Kahaleh M, Behm B, Clarke BW, Brock A, Shami VM, De La Rue SA, Sundaram V, Tokar J, Adams RB, Yeaton P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: a new paradigm? (with video) Gastrointest Endosc. 2008;67:446–454. doi: 10.1016/j.gie.2007.06.057. [DOI] [PubMed] [Google Scholar]
- 83.Sauer P, Chahoud F, Gotthardt D, Stremmel W, Weiss KH, Büchler M, Schemmer P, Weitz J, Schaible A. Temporary placement of fully covered self-expandable metal stents in biliary complications after liver transplantation. Endoscopy. 2012;44:536–538. doi: 10.1055/s-0031-1291714. [DOI] [PubMed] [Google Scholar]
- 84.Tarantino I, Traina M, Mocciaro F, Barresi L, Curcio G, Di Pisa M, Granata A, Volpes R, Gridelli B. Fully covered metallic stents in biliary stenosis after orthotopic liver transplantation. Endoscopy. 2012;44:246–250. doi: 10.1055/s-0031-1291465. [DOI] [PubMed] [Google Scholar]
- 85.Poley JW, Cahen DL, Metselaar HJ, van Buuren HR, Kazemier G, van Eijck CH, Haringsma J, Kuipers EJ, Bruno MJ. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video) Gastrointest Endosc. 2012;75:783–789. doi: 10.1016/j.gie.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 86.Kao D, Zepeda-Gomez S, Tandon P, Bain VG. Managing the post-liver transplantation anastomotic biliary stricture: multiple plastic versus metal stents: a systematic review. Gastrointest Endosc. 2013;77:679–691. doi: 10.1016/j.gie.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 87.Wang AY, Ellen K, Berg CL, Schmitt TM, Kahaleh M. Fully covered self-expandable metallic stents in the management of complex biliary leaks: preliminary data - a case series. Endoscopy. 2009;41:781–786. doi: 10.1055/s-0029-1215050. [DOI] [PubMed] [Google Scholar]
- 88.Costa-Genzini A, Takahashi W, Dos Santos RG, Gaboardi MT, Noujaim HM, Yamashita ET, Perosa M, Genzini T. Single-balloon enteroscopy for treating Roux-en-Y choledochojejunostomy stenosis after liver transplantation: a case report. Transplant Proc. 2012;44:2503–2504. doi: 10.1016/j.transproceed.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 89.Chahal P, Baron TH, Poterucha JJ, Rosen CB. Endoscopic retrograde cholangiography in post-orthotopic liver transplant population with Roux-en-Y biliary reconstruction. Liver Transpl. 2007;13:1168–1173. doi: 10.1002/lt.21198. [DOI] [PubMed] [Google Scholar]
- 90.Kawano Y, Mizuta K, Hishikawa S, Egami S, Fujiwara T, Hyodo M, Yasuda Y, Yano T, Nakazawa K, Yamamoto H, et al. Rendezvous penetration method using double-balloon endoscopy for complete anastomosis obstruction of hepaticojejunostomy after pediatric living donor liver transplantation. Liver Transpl. 2008;14:385–387. doi: 10.1002/lt.21357. [DOI] [PubMed] [Google Scholar]
- 91.Koornstra JJ, Fry L, Mönkemüller K. ERCP with the balloon-assisted enteroscopy technique: a systematic review. Dig Dis. 2008;26:324–329. doi: 10.1159/000177017. [DOI] [PubMed] [Google Scholar]
- 92.Mönkemüller K, Fry LC, Bellutti M, Neumann H, Malfertheiner P. ERCP using single-balloon instead of double-balloon enteroscopy in patients with Roux-en-Y anastomosis. Endoscopy. 2008;40 Suppl 2:E19–E20. doi: 10.1055/s-2007-966949. [DOI] [PubMed] [Google Scholar]
- 93.Sung RS, Campbell DA, Rudich SM, Punch JD, Shieck VL, Armstrong JM, Ford E, Sullivan P, Dasika NL, Magee JC. Long-term follow-up of percutaneous transhepatic balloon cholangioplasty in the management of biliary strictures after liver transplantation. Transplantation. 2004;77:110–115. doi: 10.1097/01.TP.0000101518.19849.C8. [DOI] [PubMed] [Google Scholar]
- 94.Fidelman N, Bloom AI, Kerlan RK, Laberge JM, Wilson MW, Ring EJ, Gordon RL. Hepatic arterial injuries after percutaneous biliary interventions in the era of laparoscopic surgery and liver transplantation: experience with 930 patients. Radiology. 2008;247:880–886. doi: 10.1148/radiol.2473070529. [DOI] [PubMed] [Google Scholar]
- 95.Gwon DI, Sung KB, Ko GY, Yoon HK, Lee SG. Dual catheter placement technique for treatment of biliary anastomotic strictures after liver transplantation. Liver Transpl. 2011;17:159–166. doi: 10.1002/lt.22206. [DOI] [PubMed] [Google Scholar]
- 96.Matlock J, Freeman ML. Endoscopic therapy of benign biliary strictures. Rev Gastroenterol Disord. 2005;5:206–214. [PubMed] [Google Scholar]
- 97.Chang JH, Lee IS, Chun HJ, Choi JY, Yoon SK, Kim DG, You YK, Choi MG, Choi KY, Chung IS. Usefulness of the rendezvous technique for biliary stricture after adult right-lobe living-donor liver transplantation with duct-to-duct anastomosis. Gut Liver. 2010;4:68–75. doi: 10.5009/gnl.2010.4.1.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tsukui D, Yano T, Nakazawa K, Osawa H, Kawano Y, Mizuta K, Kawarasaki H, Yamamoto H. Rendezvous technique combining double-balloon endoscopy with percutaneous cholangioscopy is useful for the treatment of biliary anastomotic obstruction after liver transplantation (with video) Gastrointest Endosc. 2008;68:1013–1015. doi: 10.1016/j.gie.2008.02.034. [DOI] [PubMed] [Google Scholar]
- 99.Greif F, Bronsther OL, Van Thiel DH, Casavilla A, Iwatsuki S, Tzakis A, Todo S, Fung JJ, Starzl TE. The incidence, timing, and management of biliary tract complications after orthotopic liver transplantation. Ann Surg. 1994;219:40–45. doi: 10.1097/00000658-199401000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Starzl TE, Putnam CW, Koep LJ. Current status of liver transplantation. South Med J. 1977;70:389–390. doi: 10.1097/00007611-197704000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kuo PC, Lewis WD, Stokes K, Pleskow D, Simpson MA, Jenkins RL. A comparison of operation, endoscopic retrograde cholangiopancreatography, and percutaneous transhepatic cholangiography in biliary complications after hepatic transplantation. J Am Coll Surg. 1994;179:177–181. [PubMed] [Google Scholar]
- 102.Thethy S, Thomson BNj, Pleass H, Wigmore SJ, Madhavan K, Akyol M, Forsythe JL, James Garden O. Management of biliary tract complications after orthotopic liver transplantation. Clin Transplant. 2004;18:647–653. doi: 10.1111/j.1399-0012.2004.00254.x. [DOI] [PubMed] [Google Scholar]
- 103.Davidson BR, Rai R, Kurzawinski TR, Selves L, Farouk M, Dooley JS, Burroughs AK, Rolles K. Prospective randomized trial of end-to-end versus side-to-side biliary reconstruction after orthotopic liver transplantation. Br J Surg. 1999;86:447–452. doi: 10.1046/j.1365-2168.1999.01073.x. [DOI] [PubMed] [Google Scholar]
- 104.Pasha SF, Harrison ME, Das A, Nguyen CC, Vargas HE, Balan V, Byrne TJ, Douglas DD, Mulligan DC. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44–51. doi: 10.1016/j.gie.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 105.Pfau PR, Kochman ML, Lewis JD, Long WB, Lucey MR, Olthoff K, Shaked A, Ginsberg GG. Endoscopic management of postoperative biliary complications in orthotopic liver transplantation. Gastrointest Endosc. 2000;52:55–63. doi: 10.1067/mge.2000.106687. [DOI] [PubMed] [Google Scholar]
- 106.Rerknimitr R, Sherman S, Fogel EL, Kalayci C, Lumeng L, Chalasani N, Kwo P, Lehman GA. Biliary tract complications after orthotopic liver transplantation with choledochocholedochostomy anastomosis: endoscopic findings and results of therapy. Gastrointest Endosc. 2002;55:224–231. doi: 10.1067/mge.2002.120813. [DOI] [PubMed] [Google Scholar]
- 107.El-Meteini M, Hamza A, Abdalaal A, Fathy M, Bahaa M, Mukhtar A, Abouelfetouh F, Mostafa I, Shaker M, Abdelwahab S, et al. Biliary complications including single-donor mortality: experience of 207 adult-to-adult living donor liver transplantations with right liver grafts. HPB (Oxford) 2010;12:109–114. doi: 10.1111/j.1477-2574.2009.00142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hwang S, Lee SG, Lee YJ, Sung KB, Park KM, Kim KH, Ahn CS, Moon DB, Hwang GS, Kim KM, et al. Lessons learned from 1,000 living donor liver transplantations in a single center: how to make living donations safe. Liver Transpl. 2006;12:920–927. doi: 10.1002/lt.20734. [DOI] [PubMed] [Google Scholar]
- 109.Iida T, Ogura Y, Oike F, Hatano E, Kaido T, Egawa H, Takada Y, Uemoto S. Surgery-related morbidity in living donors for liver transplantation. Transplantation. 2010;89:1276–1282. doi: 10.1097/TP.0b013e3181d66c55. [DOI] [PubMed] [Google Scholar]
- 110.Taketomi A, Kayashima H, Soejima Y, Yoshizumi T, Uchiyama H, Ikegami T, Yamashita Y, Harada N, Shimada M, Maehara Y. Donor risk in adult-to-adult living donor liver transplantation: impact of left lobe graft. Transplantation. 2009;87:445–450. doi: 10.1097/TP.0b013e3181943d46. [DOI] [PubMed] [Google Scholar]
- 111.Ghobrial RM, Freise CE, Trotter JF, Tong L, Ojo AO, Fair JH, Fisher RA, Emond JC, Koffron AJ, Pruett TL, et al. Donor morbidity after living donation for liver transplantation. Gastroenterology. 2008;135:468–476. doi: 10.1053/j.gastro.2008.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chan SC, Fan ST, Lo CM, Liu CL, Wong J. Toward current standards of donor right hepatectomy for adult-to-adult live donor liver transplantation through the experience of 200 cases. Ann Surg. 2007;245:110–117. doi: 10.1097/01.sla.0000225085.82193.08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lo CM. Complications and long-term outcome of living liver donors: a survey of 1,508 cases in five Asian centers. Transplantation. 2003;75:S12–S15. doi: 10.1097/01.TP.0000046534.45645.47. [DOI] [PubMed] [Google Scholar]
- 114.Brown RS, Russo MW, Lai M, Shiffman ML, Richardson MC, Everhart JE, Hoofnagle JH. A survey of liver transplantation from living adult donors in the United States. N Engl J Med. 2003;348:818–825. doi: 10.1056/NEJMsa021345. [DOI] [PubMed] [Google Scholar]
- 115.Atar E, Bachar GN, Bartal G, Mor E, Neyman H, Graif F, Belenky A. Use of peripheral cutting balloon in the management of resistant benign ureteral and biliary strictures. J Vasc Interv Radiol. 2005;16:241–245. doi: 10.1097/01.RVI.0000143767.87399.9C. [DOI] [PubMed] [Google Scholar]
- 116.Kabar I, Cicinnati VR, Beckebaum S, Cordesmeyer S, Reinecke H, Schmidt HH. Introducing paclitaxel-eluting balloons as a new treatment option of biliary-enteric anastomotic stricture after liver transplantation. Transplantation. 2012;94:e4–e5. doi: 10.1097/TP.0b013e318258d6b4. [DOI] [PubMed] [Google Scholar]
- 117.Kabar I, Cicinnati VR, Beckebaum S, Cordesmeyer S, Avsar Y, Reinecke H, Schmidt HH. Use of paclitaxel-eluting balloons for endotherapy of anastomotic strictures following liver transplantation. Endoscopy. 2012;44:1158–1160. doi: 10.1055/s-0032-1325795. [DOI] [PubMed] [Google Scholar]
- 118.Wright H, Sharma S, Gurakar A, Sebastian A, Kohli V, Jabbour N. Management of biliary stricture guided by the Spyglass Direct Visualization System in a liver transplant recipient: an innovative approach. Gastrointest Endosc. 2008;67:1201–1203. doi: 10.1016/j.gie.2007.10.055. [DOI] [PubMed] [Google Scholar]
- 119.Parsi MA, Guardino J, Vargo JJ. Peroral cholangioscopy-guided stricture therapy in living donor liver transplantation. Liver Transpl. 2009;15:263–265. doi: 10.1002/lt.21584. [DOI] [PubMed] [Google Scholar]
- 120.Ginsberg G, Cope C, Shah J, Martin T, Carty A, Habecker P, Kaufmann C, Clerc C, Nuutinen JP, Törmälä P. In vivo evaluation of a new bioabsorbable self-expanding biliary stent. Gastrointest Endosc. 2003;58:777–784. doi: 10.1016/s0016-5107(03)02016-9. [DOI] [PubMed] [Google Scholar]
- 121.Meng B, Wang J, Zhu N, Meng QY, Cui FZ, Xu YX. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J Mater Sci Mater Med. 2006;17:611–617. doi: 10.1007/s10856-006-9223-9. [DOI] [PubMed] [Google Scholar]
- 122.Tashiro H, Ogawa T, Itamoto T, Ushitora Y, Tanimoto Y, Oshita A, Amano H, Asahara T. Synthetic bioabsorbable stent material for duct-to-duct biliary reconstruction. J Surg Res. 2009;151:85–88. doi: 10.1016/j.jss.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 123.Muraoka N, Uematsu H, Yamanouchi E, Kinoshita K, Takeda T, Ihara N, Matsunami H, Itoh H. Yamanouchi magnetic compression anastomosis for bilioenteric anastomotic stricture after living-donor liver transplantation. J Vasc Interv Radiol. 2005;16:1263–1267. doi: 10.1097/01.RVI.0000173280.56442.9E. [DOI] [PubMed] [Google Scholar]
- 124.Itoi T, Yamanouchi E, Ikeuchi N, Kasuya K, Iwamoto H, Tsuchida A. Magnetic Compression Duct-to-duct Anastomosis for Biliary Obstruction in a Patient with Living Donor Liver Transplantation. Gut Liver. 2010;4 Suppl 1:S96–S98. doi: 10.5009/gnl.2010.4.S1.S96. [DOI] [PMC free article] [PubMed] [Google Scholar]


