Abstract
Objective
To characterize Medicare expenditures on initial breast cancer care and examine variation in expenditures across hospital referral regions (HRRs).
Data Source
We identified 29,110 women with localized breast cancer diagnosed in 2005–2008 and matched controls from the Surveillance, Epidemiology, and End Results‐Medicare linked database.
Study Design
Using hierarchical generalized linear models, we estimated per patient Medicare expenditure on initial breast cancer care across HRRs and assessed the contribution of patient, cancer, and treatment factors to regional variation via incremental models.
Principal Findings
Mean Medicare expenditure for initial breast cancer care was $19,255 per patient. The average expenditures varied from $15,053 in the lowest‐spending HRR quintile to $23,480 in the highest‐spending HRR quintile. Patient sociodemographic, comorbidity, and tumor characteristics explained only 1.8 percent of the difference in expenditures between the lowest‐ and highest‐spending quintiles, while use of specific treatment modalities explained 14.5 percent of the difference. Medicare spending on radiation therapy differed the most across the quintiles, with the use of intensity modulated radiation therapy increasing from 1.7 percent in the lowest‐spending quintile to 11.6 percent in the highest‐spending quintile.
Conclusions
Medicare expenditures on initial breast cancer care vary substantially across regions. Treatment factors are major contributors to the variation.
Keywords: Breast cancer, cost, variation, hospital referral region, radiation
Breast cancer is the most common malignancy in women (Centers for Disease Control and Prevention 2013). Fueled by an aging population and rapid diffusion of expensive cancer therapies, national expenditures on breast cancer totaled $16.5 billion in 2010, the highest of any cancer site (Mariotto et al. 2011). Various efforts are underway to address rising costs while maintaining or improving quality of care, such as bundled payments and episode‐based payment models (Collins et al. 2011; Goozner 2011). The Choosing Wisely campaign is also calling attention to “Top Five” tests and procedures in over 40 specialties that are expensive and commonly performed but lack clinical evidence (American Board of Internal Medicine [ABIM] Foundation 2013; Hahn et al. 2014; Schnipper et al. 2012). However, altering reimbursement and patterns of care is particularly challenging for cancer care, which often involves teams of providers from multiple specialties and numerous encounters over an extended period of time. More information on how different components of breast cancer care (e.g., surgery, radiation, systemic therapy, growth factors, imaging tests) influence the overall costs can help determine the appropriate payment amount and prioritize target areas in order for these efforts to have an impact on costs.
In addition, although prior work has documented regional variation in breast cancer care costs, factors that contribute to this variation remain unknown (Brooks et al. 2013). Identifying modifiable and nonmodifiable contributors to geographic variation can help to understand and predict resource needs at the population level, and it may also suggest opportunities for cost reduction. For instance, variation in costs may be due to differences in patient sociodemographics (e.g., age, race/ethnicity, and urban/rural location) and comorbidity across regions (Zuckerman et al. 2010; White 2012). Alternatively, tumor characteristics could be the major determinant of cost variation, as earlier stage patients tend to accrue lower expenditures (Yabroff et al. 2008). Understanding the role of these patient characteristics in explaining spending variation can enhance risk adjustment models to more appropriately determine payment rates in provider payment reforms (e.g., bundled payment).
Cancer treatment factors (e.g., provider practice style) may also contribute to regional variation in costs of breast cancer care. Both the type of therapies used (e.g., surgery, radiation therapy, chemotherapy) and the specific modalities used, especially the newer, more expensive modalities (e.g., intensity modulated radiation therapy [IMRT], brachytherapy), can have important cost implications. For example, recent studies demonstrated rapid adoption of IMRT and brachytherapy in Medicare beneficiaries with breast cancer (Presley et al. 2012; Roberts et al. 2013). However, evidence on different utilization rates for these specific modalities across hospital referral regions (HRRs) (Presley et al. 2012; Roberts et al. 2013) may suggest locales with under‐ or overutilization of these modalities. Identifying modifiable treatment factors that contribute the most to regional variation in breast cancer care costs can inform strategies for containing costs and improving efficiency.
To shed light on these issues, we characterized Medicare expenditures on initial cancer care for older women diagnosed with localized breast cancer (both total expenditure and expenditures on different components of care). We also examined variation in breast cancer expenditures across HRRs and the contribution of patient sociodemographic, comorbidity, and tumor characteristics, as well as treatment factors, to the overall expenditures and observed regional variation.
Methods
Data Sources and Study Sample
This was a retrospective cohort study using the 2003–2009 Surveillance, Epidemiology, and End Results (SEER)‐Medicare linked database. We identified women age 67 and older who were diagnosed with invasive, stage I–III breast cancer during 2005 through 2008. Data during a 2‐year period prior to diagnosis were used to assess comorbidity status, while data during the year after diagnosis were used to measure expenditures. We used each beneficiary's residence location to link women to HRRs (Dartmouth Atlas of Health Care 2011).
Each woman identified with breast cancer (“case”) was matched to three women without cancer (“control”) using noncancer patients from the 5 percent random sample of Medicare beneficiaries residing in the SEER regions. An index date for controls was assigned as the first day of a randomly selected month in which the control was alive and met the same enrollment criteria used for cases except for cancer diagnosis. Exact matching was performed based on HRR, year of diagnosis/index date, age, comorbidity, and precancer Medicare expenditure (from 18 through 6 months prior to diagnosis/index date to avoid expenditures associated with work‐up leading to cancer diagnosis).
We excluded patients who had missing data on tumor stage, had a history of a prior or concurrent malignancy, could not be linked to an HRR based on zip code, did not have continuous Medicare Parts A and B coverage, or were enrolled in a managed care plan during their observation period. The Yale Human Investigation Committee determined that this study did not directly involve human subjects.
Measures
Consistent with prior studies (Warren et al. 2008; Yabroff et al. 2009), we defined initial phase of care as the period from 2 months prior to through 12 months after breast cancer diagnosis/index date. For each beneficiary, we calculated Medicare expenditures as the sum of all Medicare payments over the initial phase of care, including payments for inpatient, physician, hospital‐based outpatient, home health, durable medical equipment, and hospice care based on claims data. We adjusted for geographic variation in input prices across SEER regions (Centers for Medicare and Medicaid Services 2013c,d) and reported all expenditures in 2009 U.S. dollars (Centers for Medicare and Medicaid Services 2013e). For cases only, we also separately assessed Medicare expenditures on surgery, radiation therapy, systemic therapy, growth factors, and imaging services over the same time period (Appendix SA2).
We searched for comorbid conditions using International Classification of Diseases, 9th revision (ICD‐9) diagnosis codes appearing on an inpatient claim or at least two outpatient claims billed more than 30 days apart during 24 through 3 months prior to the diagnosis/index date. Patients were coded as having 0, 1–2, or at least 3 conditions using a modified list of conditions suggested by Elixhauser et al. (1998). Other measures, including patient age (as of diagnosis/index date), race, and tumor characteristics, were directly obtained from the SEER‐Medicare database and the 5 percent random sample of noncancer Medicare beneficiaries for cases and controls.
Statistical Analysis
To account for the right skewed data while accommodating the clustering of patients within HRRs, we used hierarchical generalized linear models (HGLMs) with log link and Poisson distribution. We selected the Poisson distribution based on a modified Park test (Manning and Mullahy 2001) after we winsorized the data at the upper 99th percentile to reduce the influence of extreme values (Dixon 1974; Jha et al. 2009; Adams et al. 2010). We jointly modeled Medicare expenditures of cases and controls within a single HGLM model by including separate intercepts for cases and controls and specifying separate random effects for cases and controls across HRRs while allowing the two random effects from the same HRR to be correlated.
To assess the relative contribution of patient, cancer, and treatment factors to reducing regional variation in total expenditures, we estimated a series of HGLM models by adding these factors to the model sequentially. Model 0 only included the case and control indicators, and their random effects. Model 1 also adjusted for age, race, metropolitan statistical area status, median household income, comorbidity, and year of diagnosis/index date (Appendix SA3), while Model 2 additionally adjusted for cancer stage, laterality, hormone receptor status, and lymph node involvement. Finally, we examined the impact of treatment factors, that is, whether different treatment modalities were used (breast conserving surgery, mastectomy, IMRT, brachytherapy, external beam radiation therapy [EBRT], biological therapy, chemotherapy, growth factors, and imaging) (Model 3). As covariance between the two random effects for cases and controls was not significantly different from zero after patient matching variables were adjusted for, we omitted it in models 1–3 to reduce computational burden in model estimation. In addition, we included interaction terms between age categories and the case/control indicator in models 1–3 to account for heterogeneity in the effects of age on expenditures between cases and controls. We excluded 11 patients with unknown laterality from all regression models due to difficulty of including rare risk factors and the desire to maintain a consistent cohort across models.
In all analyses, we estimated the mean or mean adjusted expenditure for cases and controls in a given HRR, and calculated their difference as being “cancer‐related.” This approach removed noncancer costs while allowing us to use model adjustment for patient covariates. Following each model, we calculated for each patient her “predicted” Medicare expenditure (i.e., estimated expenditure conditional on the HRR's specific random effects) and “expected” Medicare expenditure (i.e., estimated expenditure conditional on the HRR random effects being zero). Then, for a given HRR i, we estimated its risk‐standardized, per patient Medicare expenditure on breast cancer care as:
where and are the mean predicted Medicare expenditure over all cases and controls, respectively, in HRR i; and are the mean expected Medicare expenditure over all cases and controls, respectively, in HRR i; and and are the mean observed Medicare expenditure calculated over all cases and controls, respectively, in the entire sample (Timbie and Normand 2008).
After each model, we assigned HRRs to quintiles according to their risk‐standardized, per patient Medicare expenditure on breast cancer care derived from the model. To ensure robust quintile assignment, we simulated a distribution of risk‐standardized expenditures for each HRR using the posterior distributions of the two random effects. Specifically, for each HRR, we generated 1,000 pairs of random effects (case and control) using the posterior mean and standard error for each effect and their covariance (when applicable) from the model. For each pair of simulated random effects, we calculated the risk‐standardized expenditures as indicated in the equation above for each HRR and classified HRRs into quintiles. The final assignment of each HRR was based on its mode (i.e., the most common) quintile from the 1,000 simulations.
To estimate observed, per patient Medicare breast cancer–related expenditure for each HRR, we calculated the difference in mean observed Medicare expenditure between women with breast cancer and those without cancer. We reported its distribution across HRR quintiles using quintiles determined by Model 0. We also examined the distribution of mean observed Medicare expenditures on different components of care (i.e., expenditures on surgery, radiation therapy, systemic therapy, growth factors, and imaging services) across these quintiles. The extent of regional variation was assessed by the difference in expenditures between the highest‐ and the lowest‐spending quintiles.
The relative contribution of patient, cancer, and treatment factors to reducing regional variation in breast cancer expenditures was examined by comparing differences in per patient mean expenditures on breast cancer‐related care between the highest‐ and the lowest‐spending quintiles across Models 0–3. HRR quintiles were reassigned after each model to appropriately reflect the remaining variation in breast cancer–related expenditures.
While cases and controls from all HRRs were included in estimating the risk adjustment models, we limited our HRR level analysis to HRRs with at least 25 cancer patients to ensure reliable estimates. All data management (including the matching of cases and controls) and analysis were performed using SAS 9.4 (SAS Inc, Cary, NC, USA) and Stata 13 (StataCorp, College Station, TX, USA).
Results
Sample Characteristics
There were 29,110 women with stage I–III breast cancer in our cohort, residing in 99 HRRs (Table 1). Over half (55.4 percent) were diagnosed at stage I, and 33.3 and 11.3 percent were diagnosed at stage II and stage III, respectively. Sixty percent of the patients had tumors less than 2 centimeters, and 80.1 percent had hormone receptor–positive breast cancer. After excluding patients with missing data, 89 HRRs had at least 25 cases.
Table 1.
Characteristics of Breast Cancer Patients
| Sample Characteristics | Breast Cancer Cases | Controls |
|---|---|---|
| N (%) | N (%) | |
| Sample size | 29,110 (100.0) | 87,330 (100.0) |
| Age at diagnosisa, in years | ||
| 67–69 | 4,990 (17.1) | 15,035 (17.2) |
| 70–74 | 7,585 (26.1) | 22,631 (25.9) |
| 75–79 | 7,016 (24.1) | 20,846 (23.9) |
| 80–84 | 5,595 (19.2) | 15,319 (17.5) |
| 85–94 | 3,924 (13.5) | 13,499 (15.5) |
| Race | ||
| White | 25,823 (88.7) | 72,561 (83.1) |
| Black | 2,031 (7.0) | 7,118 (8.2) |
| Other | 1,256 (4.3) | 7,651 (8.8) |
| Elixhauser comorbid score | ||
| No conditions | 12,696 (43.6) | 38,088 (43.6) |
| 1–2 conditions | 11,450 (39.3) | 32,675 (37.4) |
| ≥3 conditions | 4,964 (17.1) | 16,567 (19.0) |
| Metropolitan statistical area | ||
| Yes | 4,690 (16.1) | 14,533 (16.6) |
| No | 24,420 (83.9) | 72,797 (83.4) |
| Median household income | ||
| <$33,000 | 5,863 (20.1) | 17,338 (19.9) |
| $33,000–$39,999 | 4,293 (14.7) | 14,674 (16.8) |
| $40,000–$49,999 | 6,077 (20.9) | 18,841 (21.6) |
| $50,000–$62,999 | 5,750 (19.8) | 16,948 (19.4) |
| ≥$63,000 | 7,122 (24.5) | 16,626 (19.0) |
| Year at diagnosisa | ||
| 2005 | 7,141 (24.5) | 21,423 (24.5) |
| 2006 | 7,236 (24.9) | 21,708 (24.9) |
| 2007 | 7,429 (25.5) | 22,287 (25.5) |
| 2008 | 7,304 (25.1) | 21,912 (25.1) |
| Baseline annual Medicare expenditureb | ||
| Median (interquartile range) ($) | $2,079 ($745–$5,467) | $2,080 ($745–$5,474) |
| Breast cancer stage | ||
| Stage I | 16,117 (55.4) | |
| Stage II | 9,707 (33.3) | |
| Stage III | 3,286 (11.3) | |
| Tumor size | ||
| <2 cm | 17,569 (60.4) | |
| 2–5 cm | 9,635 (33.1) | |
| >5 cm | 1,538 (5.3) | |
| Missing | 368 (1.3) | |
| Laterality | ||
| Left | 14,780 (50.8) | |
| Right | 14,319 (49.2) | |
| Other/unknown | 11 (0.0) | |
| Lymph node involvement | ||
| Yes | 7,119 (24.5) | |
| No/unknown | 21,991 (75.5) | |
| Hormone receptor statusc | ||
| Positive | 23,312 (80.1) | |
| Negative | 4,047 (13.9) | |
| Unknown | 1,751 (6.0) | |
Percentages may not add to 100% due to rounding.
Measured at the time of diagnosis (for cancer patients) or index date (for controls).
Assessed over a 12‐month period from 18 through 6 months prior to diagnosis/index date to avoid cost associated with work‐up leading to cancer diagnosis.
Estrogen or progesterone.
Medicare Expenditures on Initial Breast Cancer Treatment
Based on our net costing method that estimated breast cancer–related expenditure as the difference in mean per person expenditure between cases and controls, the estimated per patient Medicare expenditure for the initial treatment of breast cancer averaged $19,255 (Table 2). Surgery, radiation therapy, and systemic therapy accounted for the largest shares of the expenditures, comprising 33.9 percent ($6,527), 30.3 percent ($5,828), and 14.0 percent ($2,702) of the total expenditures, respectively. Growth factors and imaging services only accounted for 8.1 percent ($1,558) and 4.3 percent ($831) of the total expenditures, respectively. The remaining 9.4 percent of the expenditure was for “other” care that was not included in these main treatment categories, such as complications of treatments and nonimaging lab tests.
Table 2.
Medicare Expenditures on Breast Cancer–Related Care across Hospital Referral Regions (HRRs)
| Cost Component | All HRRs | HRR Quintiles, Based on Average per Patient Medicare Expenditure on Initial Breast Cancer Care | Difference between Quintile 1 and Quintile 5 | |||||
|---|---|---|---|---|---|---|---|---|
| Mean | Standard Deviation | 1 (Lowest) | 2 | 3 | 4 | 5 (Highest) | ||
| Total expenditure—cases | $29,452 | $3,773 | $24,617 | $28,077 | $29,861 | $31,207 | $34,062 | $9,445 |
| Total expenditure—controls | $10,200 | $1,889 | $9,546 | $10,328 | $10,365 | $10,203 | $10,584 | $1,038 |
| Total breast cancer–related expenditurea | $19,255 | $2,977 | $15,053 | $17,760 | $19,525 | $20,993 | $23,480 | $8,427 |
| Surgery | $6,527 | $755 | $6,074 | $6,299 | $6,634 | $6,736 | $6,939 | $865 |
| Radiation therapy | $5,828 | $1,389 | $5,066 | $5,432 | $5,624 | $6,389 | $6,790 | $1,724 |
| Systemic therapy | $2,702 | $911 | $2,017 | $2,722 | $2,631 | $2,974 | $3,247 | $1,230 |
| Growth factors | $1,558 | $602 | $1,138 | $1,520 | $1,456 | $1,752 | $1,997 | $859 |
| Imaging services | $831 | $246 | $713 | $777 | $855 | $922 | $898 | $185 |
| Other expenditures | $1,805 | $1,599 | $63 | $1,000 | $2,297 | $2,231 | $3,605 | $3,542 |
Expenditures reflect inflation adjusted constant 2009 U.S. dollars and after removing regional differences in input prices. HRR quintiles were assigned based on simulated results from a hierarchical generalized linear regression model with no covariates but separate intercepts for cases and controls and HRR random effects for cases and controls to account for clustering in data within HRRs (Model 0).
For each HRR, the average per person expenditure on breast cancer–related care reflects the difference in mean expenditure between cases and controls.
Across the HRRs, the average per patient expenditure on breast cancer–related care (based on the net difference in mean expenditures between cases and controls) was $15,053 in the lowest‐spending quintile, compared to $23,480 in the highest‐spending quintile, reflecting a 56.0 percent increase between the lowest and highest quintiles (Table 2). Of the treatment categories examined, Medicare spending on radiation therapy differed the most across the quintiles, rising from $5,066 in the lowest‐spending quintile to $6,790 in the highest‐spending quintile. Differences in Medicare expenditures on other treatment categories were smaller (Table 2).
Factors Contributing to Regional Variation in Breast Cancer Expenditures
The unadjusted model (Model 0) showed a difference of $8,292 in per patient breast cancer expenditures between HRRs in the lowest‐ and highest‐spending quintiles (Figure 1). Adjustment for patient sociodemographics and comorbidity (Model 1) and tumor characteristics (Model 2) reduced the difference in risk‐standardized expenditures between the lowest‐ and highest‐spending quintiles by 1.1 and 0.7 percent, respectively, with a total of 1.8 percent of the difference being explained. Adding binary indicators for the specific treatment modalities (e.g., IMRT, brachytherapy, EBRT, biological therapy, chemotherapy) (Model 3) reduced the difference in expenditure between the lowest‐ and highest‐spending quintiles to $6,936 (i.e., a 14.5 percent reduction on top of Model 2). Hence, adjustment for patient, cancer, and treatment characteristics together explained 16.4 percent of the difference in Medicare expenditures on initial breast cancer care between the lowest‐ and the highest‐spending quintiles.
Figure 1.
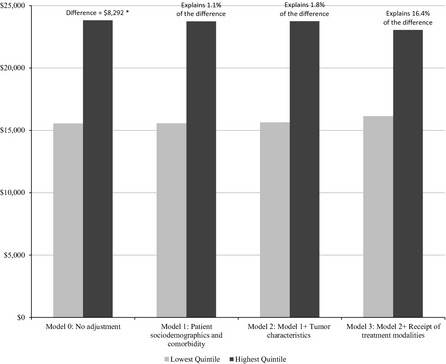
Variation in Medicare Breast Cancer Expenditures between the Lowest‐ and Highest‐Spending Quintiles of Hospital Referral Regions (HRRs), after Adjustment of Selected Patient, Cancer, and Treatment Factors in Sequential Models
- Note. The percentages reflect the proportion of the initial difference in per patient Medicare expenditure on breast cancer care between the lowest‐ and highest‐spending quintiles explained by each model. *This number differs slightly from the difference in average per patient total breast cancer–related Medicare expenditure between quintile 1 and quintile 5 reported in Table 2. The number in Table 2 (i.e., $8,427) was based on observed expenditures, while the number reported in this figure (i.e., $8,292) was based on a hierarchical generalized linear regression model with no covariates but separate intercepts for cases and controls and HRR random effects for cases and controls to account for clustering in data within HRRs (Model 0).
Of the treatments examined, utilization of radiation therapy varied the most across the HRRs, with the higher spending quintiles more likely to use the newer, more expensive therapies (Table 3). For example, the proportion of patients receiving IMRT increased from 1.7 percent in the lowest‐spending quintile to 11.6 percent in the highest‐spending quintile, while the proportion using EBRT decreased slightly across the quintiles. Variation in the receipt of other treatments was less striking. In addition, among patients who received a given type of treatment, the average expenditure on radiation therapy differed the most between HRRs in the lowest‐ and highest‐spending quintiles ($10,314 vs. $12,508).
Table 3.
Utilization of Breast Cancer Treatment Modalities across Hospital Referral Regions (HRRs)
| Treatment Modality | HRR Quintiles, Based on Average per Patient Medicare Expenditure on Initial Breast Cancer Care | Difference between Quintile 1 and Quintile 5 | ||||
|---|---|---|---|---|---|---|
| 1 (Lowest) | 2 | 3 | 4 | 5 (Highest) | ||
| Proportion of patients receiving each treatment modality (%) | ||||||
| Surgery | 97.7 | 97.9 | 98.5 | 97.8 | 98.6 | 0.9 |
| Radiation therapy | 49.8 | 49.3 | 52.6 | 53.0 | 54.0 | 4.2 |
| EBRT | 43.7 | 40.2 | 41.3 | 36.6 | 37.0 | −6.7 |
| IMRT | 1.7 | 4.5 | 6.7 | 10.8 | 11.6 | 9.9 |
| Brachytherapy | 3.3 | 3.9 | 3.8 | 4.9 | 4.3 | 1.0 |
| Systemic therapy | 16.4 | 19.9 | 19.6 | 21.8 | 24.4 | 8.0 |
| Biologic therapy | 3.7 | 5.0 | 5.0 | 4.8 | 5.8 | 2.1 |
| Chemotherapy | 16.0 | 19.7 | 19.4 | 21.6 | 23.9 | 7.9 |
| Growth factors | 11.8 | 15.5 | 15.1 | 16.8 | 18.9 | 7.1 |
| Imaging services | 97.0 | 96.8 | 96.8 | 96.8 | 96.7 | −0.3 |
| Average spending among patients who received a given treatment modality | ||||||
| Surgery | $6,214 | $6,437 | $6,735 | $6,887 | $7,034 | $820 |
| Radiation therapy | $10,314 | $10,990 | $10,646 | $12,002 | $12,508 | $2,194 |
| Systemic therapy | $12,806 | $13,721 | $13,309 | $13,530 | $13,204 | $398 |
| Growth factors | $9,799 | $9,811 | $9,735 | $10,333 | $10,271 | $472 |
| Imaging services | $735 | $802 | $883 | $953 | $930 | $195 |
HRR quintiles were assigned based on simulated results from a hierarchical generalized linear regression model with no covariates but separate intercepts for cases and controls and HRR random effects for cases and controls to account for clustering in data within HRRs (Model 0).
EBRT, external beam radiation therapy; IMRT, intensity modulated radiation therapy.
Discussion
Medicare expenditure on initial breast cancer care amounted to nearly $20,000 per patient during the initial course of treatment. It varied substantially across HRRs, ranging from $15,053 in the lowest‐spending quintile to $23,480 in the highest‐spending quintile. Patient sociodemographic, comorbidity, and tumor characteristics explained little of the variation, whereas treatment factors, particularly the more expensive IMRT therapy, were important contributors to the regional variation. However, considerable variation remained after adjustment for these factors, highlighting the need for additional research to discern the causes of variation.
Our study extends prior research on breast cancer costs in several important ways. First, we assessed contributors to total spending and showed that in addition to surgery, radiation therapy and systemic therapy accounted for the largest shares. Initiatives that can identify and reduce the use of “unnecessary” procedures in these areas would be most effective in lowering the costs of breast cancer care. Bundling payments in these services may be an effective strategy to improve efficiency. Conversely, growth factors and imaging services only accounted for 8.1 and 4.3 percent of the total expenditures, respectively. Inclusion of imaging with PET, CT, and radionuclide bone scans in the American Society of Clinical Oncology's “Top Five” list of questionable oncology services may provide relatively little opportunity for cost reduction in women with localized breast cancer (Schnipper et al. 2012).
Our study also provided important new insights about regional variation in breast cancer costs. Prior studies demonstrated a two‐fold difference in age‐standardized Medicare expenditures on breast cancer screening across HRRs (from $42 to $107 per beneficiary) (Gross et al. 2013), and a nearly $10,000 difference in breast cancer treatment between the lowest‐ and highest‐spending HRR quintiles for women with stage IV breast cancer (over a 6‐month period after diagnosis) (Brooks et al. 2013). Our analysis also found large variation in Medicare expenditures across HRRs in women with localized breast cancer. More important, we demonstrated that commonly invoked factors such as patient case mix explained little of this variation. Contrary to other studies showing that demographic and baseline health characteristics explain 33 percent of the difference in per‐beneficiary spending between the highest and lowest HRR quintiles in the general Medicare population (Zuckerman et al. 2010), we found that variation in sociodemographics and comorbidity only explained 1.1 percent of the difference in Medicare breast cancer‐related expenditures between the highest and lowest quintiles. This may be due to our use of net costing method with matched cases and controls, and hence the remaining influence of the sociodemographics and comorbidities reflects their effect on breast cancer spending specifically. These results suggest that evidence on sources of cost variation derived from other populations may not apply to breast cancer care.
Cancer treatment factors, rather than tumor characteristics, are the more important underlying drivers of regional spending variation. Adjustment for breast cancer tumor characteristics only reduced another 0.7 percent of the difference in Medicare expenditures between the highest and the lowest quintiles. In contrast, treatment factors explained 14.5 percent of the difference in Medicare expenditures between the highest and the lowest quintiles in our analysis. Radiation therapy was a particularly important contributor to variation across regions. Higher spending HRRs used the more expensive modalities, particularly IMRT, more frequently than lower spending HRRs. This is consistent with recent studies documenting wide variation in IMRT and brachytherapy use across geographic regions (Presley et al. 2012; Roberts et al. 2013). As one method to improve radiation dose homogeneity, breast IMRT has been shown to reduce skin reactions and soft tissue fibrosis (Donovan et al. 2007; Pignol et al. 2008). However, the cost effectiveness of breast IMRT is yet to be established given the availability of other less costly techniques with similar skin and cosmetic benefits (Haffty, Buchholz, and McCormick 2008; Kachnic and Powell 2011; Roof and Marks 2014; Sen et al. 2014). Indeed, the “Top Five” list identified by the American Society for Radiation Oncology also questions routine use of IMRT for whole breast radiotherapy as part of breast conservation therapy (Hahn et al. 2014). Therefore, optimizing practices surrounding radiation therapy, particularly IMRT, could be an effective strategy to address variation in Medicare spending. Comparative effectiveness and cost effectiveness research is needed to fully assess the benefit of the more expensive treatment approaches in breast cancer care and identify patient groups who may benefit the most from its use. If evidence does not support their benefit or widespread use, payment reforms (e.g., bundled payment to incentivize the provision of more cost effective radiation therapy, or targeting use of IMRT toward subgroups that are likely to benefit from it) may be warranted. In addition, the current reimbursement system in the United States does not appropriately distinguish the varying level of complexity in the IMRT techniques, which can be another area of potential reforms (e.g., vary reimbursement rates depending on specific IMRT techniques used). Moreover, although difference in screening related factors such as stage at diagnosis is not a main determinant of cost variation across geographic regions in elderly breast cancer patients, the importance of these factors should not be dismissed altogether as they may influence other forms of variation such as race‐based differences in costs and patient outcomes.
While we do not know the exact causes for the observed difference in use of radiation therapy across HRRs, prior studies suggest that availability of specialists and regional practice patterns might have played some role. Feinstein et al. (2013) showed that among patients with early‐stage breast cancer and short life expectancy, who are less likely to benefit from adjuvant radiation therapy, the risk‐standardized rate of adjuvant radiation therapy was significantly higher in HRRs with high density of radiation oncologists. Presley et al. (2012) found regional differences in receipt of brachytherapy, with higher utilization in the east coast and southwestern part of the country. Additional research linking characteristics of geographic regions to radiation therapy use, as well as more in‐depth comparison of low‐ versus high‐utilization regions, would help identify underlying drivers for regional difference in radiation use.
Nevertheless, considerable regional variation (83.6 percent of the difference in total expenditure between the lowest‐ and highest‐spending HRR quintiles) remained unexplained after accounting for patient characteristics and treatment factors. This is consistent with findings from Zuckerman et al. (2010) showing that 63 percent of the geographic variation in Medicare spending remain unaccounted for after adjusting for differences in beneficiaries' sociodemographic characteristics, health status, and area supply of health care resources. Continued effort is needed to identify other important drivers of the observed variation in spending, such as provider and regional characteristics and potential differences in intensity of therapies and treatment complications across HRRs. It is also likely that local areas or care networks within HRRs have substantial variation as well, which may confound HRR level variation (Zhang et al. 2012). Further research on factors that explain differences in breast cancer spending across these more refined units of care delivery may provide additional insights.
Finally, building upon previous episode‐based expenditure measures for breast cancer (Collins et al. 2011), our study developed an analytic framework that incorporates state‐of‐the‐art modeling techniques for risk adjustment and allows for a rigorous case–control design. Consistent with current approaches to assess risk‐standardized outcomes used by the Centers for Medicare and Medicaid Services (2013a,b), our method was developed to meet the unique needs of cost analysis and cancer research. The net costing method enabled us to estimate breast cancer expenditures without subjective assumptions about which procedures are cancer‐related or not (Barlow 2009). Compared to the adjustment method used in other studies that relied on a weighted average of patient case mix strata (Brooks et al. 2013), the HGLM risk adjustment models account for the correlation of expenditures within the same HRRs and provide flexibility in incorporating a larger number of risk factors. Our approach can be readily applied to study other cancer sites, facilitating future cancer research and development of episode‐based payment measure in cancer care.
Several limitations of the study should be acknowledged. First, our analysis was based on SEER‐Medicare data, so our findings may not be generalizable to younger patients or patients residing outside SEER regions. Second, our measurement of service utilization and comorbid conditions relied on claims data, which may lack sufficient clinical detail or be subject to errors or omissions in billing codes. Third, we were not able to include expenditures on hormone therapy in our analysis because we did not have Medicare Part D data for the entire study period. Given that 80.1 percent of the women in our sample were hormone receptor positive, future research examining the contribution of hormone therapy to overall expenditures and regional variation in expenditures is needed. Fourth, our use of the net costing method relied on the quality of matching between cases and controls and an inherent assumption that data from the controls can adequately reflect noncancer care expenditure for cases in the same HRR. Although our data support good quality of matching in our sample, it remains possible that our identified variation in breast cancer–related care across HRRs may be confounded by differences in consumption of medical resources among the controls across geographic region. In addition, if cancer patients tend to defer treatment for noncancer conditions, our method may overestimate expenditures on noncancer care and hence underestimate cancer‐related expenditures. Fifth, our analysis was limited to Medicare expenditures, which only reflect a portion of the patient's overall health care expenditures (Yabroff et al. 2009). Replication of our analysis using other data sources with more comprehensive measurement of resource utilization (e.g., including patient out‐of‐pocket expenditure and payment by other insurers) will provide additional insights. Finally, we recognize that by focusing on expenditures alone, the current study cannot fully inform policy discussion on value of care. Future research assessing the linkage between expenditures and patient outcomes across regions is needed.
In summary, we found that Medicare expenditures for women with localized breast cancer were substantial, and that surgery, radiation therapy, and systemic therapy contributed the most to the overall expenditures. In contrast, growth factors and imaging services only accounted for small proportions of the total expenditures. We also demonstrated large regional variation in breast cancer expenditures that was not explained substantively by patient comorbidity and tumor characteristics. Instead, cancer‐specific treatment factors, particularly the use of different radiation modalities, were found to be important contributors to the variation. Comparative effectiveness research focusing on these treatment factors can inform future cost containment initiatives. Nonetheless, substantial regional variation remained even after adjustment for patient and treatment factors, highlighting the need for additional research to identify underlying causes for the variation. In addition, understanding how variations in health care spending may be associated with patient outcomes is another important next step to inform value of breast cancer care.
Supporting information
Appendix SA1: Author Matrix.
Appendix SA2: Current Procedural Technology (CPT) Codes and Healthcare Common Procedure Coding System (HCPCS) Codes Used to Identify Claims Corresponding to Surgery, Radiation Therapy, Systemic Therapy, Growth Factors, and Imaging Services.
Appendix SA3: Risk Factor Estimates for Total Medicare Expenditures for Cases and Controls (Expenditures Measured in $1,000s).
Acknowledgments
Joint Acknowledgment/Disclosure Statement: This research was supported by grant 5R01CA149045 (Cary Gross, PI) from the National Cancer Institute. This study was presented at the 2013 San Antonio Breast Cancer Symposium in San Antonio, Texas; December 14, 2013.
Disclosures: We disclose that Ms. Soulos receives research funding from 21st Century Oncology, Dr. Ma served as a consultant for Celgene Corp., and Dr. Gross serves as a Scientific Advisory Board member for FAIR Health, Inc. and receives research funding from Medtronic, Inc., Johnson and Johnson, and 21st Century Oncology.
Disclaimers: The sponsor had no role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; in the preparation, review, or approval of the manuscript; or in the decision to submit the manuscript for publication.
References
- Adams, J. L. , Mehrotra A., Thomas J. W., and McGlynn E. A.. 2010. “Physician Cost Profiling–Reliability and Risk of Misclassification.” New England Journal of Medicine 362 (11): 1014–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Board of Internal Medicine (ABIM) Foundation . 2013. “Choosing Wisely® ” [accessed on November 24, 2013]. Available at http://www.choosingwisely.org/
- Barlow, W. E. 2009. “Overview of Methods to Estimate the Medical Costs of Cancer.” Medical Care 47 (7 Suppl 1): S33–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks, G. A. , Li L., Sharma D. B., Weeks J. C., Hassett M. J., Yabroff K. R., and Schrag D.. 2013. “Regional Variation in Spending and Survival for Older Adults with Advanced Cancer.” Journal of the National Cancer Institute 105 (9): 634–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention . 2013. “Cancer among Women” [accessed on September 25, 2013]. Available at http://www.cdc.gov/cancer/dcpc/data/women.htm
- Centers for Medicare and Medicaid Services . 2013a. “Measure Methodology Reports: Mortality Measures” [accessed on October 8, 2013]. Available at https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1163010421830
- Centers for Medicare and Medicaid Services . 2013b. “Measure Methodology Reports: Readmission Measures” [accessed on October 8, 2013]. Available at https://www.qualitynet.org/dcs/ContentServer?c=Page&pagename=QnetPublic%2FPage%2FQnetTier4&cid=1219069855841
- Centers for Medicare and Medicaid Services . 2013c. PFS Relative Value Files. Baltimore, MD: Centers for Medicare & Medicaid Services; [accessed on February 21, 2013]. Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/PhysicianFeeSched/PFS-Relative-Value-Files.html [Google Scholar]
- Centers for Medicare and Medicaid Services . 2013d. Wage Index Files. Baltimore, MD: Centers for Medicare & Medicaid Services; [accessed on February 21, 2013]. Available at http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Wage-Index-Files.html [Google Scholar]
- Centers for Medicare and Medicaid Services . 2013e. Market Basket Data. Baltimore, MD: Centers for Medicare and Medicaid Services; [accessed on February 21, 2013]. Available at http://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/MedicareProgramRatesStats/MarketBasketData.html [PubMed] [Google Scholar]
- Collins, E. D. , Craft M., Endsley S., Kurtzman S., McGinty G., Neuss M., Swegler E., Wallner P., Wilhoit C., and Willey S.. 2011. Episode‐of‐Care for Treatment in Newly Diagnosed Cases of Breast Cancer over a 15‐Month Period. Chicago, IL: American Board of Medical Specialties. [Google Scholar]
- Dartmouth Atlas of Health Care . 2011. Downloads, Crosswalks, Zip Code Crosswalks 1995–2010. Lebanon, NH: The Dartmouth Institute for Health Policy and Clinical Practice. [Google Scholar]
- Dixon, W. J. 1974. “Trimming and Winsorization: A Review.” Statistical Papers 14 (2–3): 157–70. [Google Scholar]
- Donovan, E. , Bleakley N., Denholm E., Evans P., Gothard L., Hanson J., Peckitt C., Reise S., Ross G., Sharp G., Symonds‐Tayler R., Tait D., and Yarnold J.; Breast Technology Group . 2007. “Randomised Trial of Standard 2D Radiotherapy (RT) versus Intensity Modulated Radiotherapy (IMRT) in Patients Prescribed Breast Radiotherapy.” Radiotherapy and Oncology 82 (3): 254–64. [DOI] [PubMed] [Google Scholar]
- Elixhauser, A. , Steiner C., Harris D. R., and Coffey R. M.. 1998. “Comorbidity Measures for Use with Administrative Data.” Medical Care 36 (1): 8–27. [DOI] [PubMed] [Google Scholar]
- Feinstein, A. J. , Soulos P. R., Long J. B., Herrin J., Roberts K. B., Yu J. B., and Gross C. P.. 2013. “Variation in Receipt of Radiation Therapy after Breast‐Conserving Surgery: Assessing the Impact of Physicians and Geographic Regions.” Medical Care 51 (4): 330–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goozner, M. 2011. “United Healthcare, Five Oncology Practices Try Bundled Payments.” Journal of the National Cancer Institute 103 (1): 8–10. [DOI] [PubMed] [Google Scholar]
- Gross, C. P. , Long J. B., Ross J. S., Abu‐Khalaf M. M., Wang R., Killelea B. K., Gold H. T., Chagpar A. B., and Ma X.. 2013. “The Cost of Breast Cancer Screening in the Medicare Population.” JAMA Internal Medicine 173 (3): 220–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haffty, B. G. , Buchholz T. A., and McCormick B.. 2008. “Should Intensity‐modulated Radiation Therapy Be the Standard of Care in the Conservatively Managed Breast Cancer Patient?” Journal of Clinical Oncology 26 (13): 2072–4. [DOI] [PubMed] [Google Scholar]
- Hahn, C. , Kavanagh B., Bhatnagar A., Jacobson G., Lutz S., Patton C., Potters L., and Steinberg M.. 2014. “Choosing Wisely: The American Society for Radiation Oncology's Top 5 List.” Pract Radiat Oncol 4 (6): 349–55. [DOI] [PubMed] [Google Scholar]
- Jha, A. K. , Orav E. J., Dobson A., Book R. A., and Epstein A. M.. 2009. “Measuring Efficiency: The Association of Hospital Costs and Quality of Care.” Health Affairs (Millwood) 28 (3): 897–906. [DOI] [PubMed] [Google Scholar]
- Kachnic, L. A. , and Powell S. N.. 2011. “IMRT for Breast Cancer–Balancing Outcomes, Patient Selection, and Resource Utilization.” Journal of the National Cancer Institute 103 (10): 777–9. [DOI] [PubMed] [Google Scholar]
- Manning, W. G. , and Mullahy J.. 2001. “Estimating Log Models: To Transform or Not to Transform?” J Health Econ 20 (4): 461–94. [DOI] [PubMed] [Google Scholar]
- Mariotto, A. B. , Yabroff K. R., Shao Y., Feuer E. J., and Brown M. L.. 2011. “Projections of the Cost of Cancer Care in the United States: 2010‐2020.” Journal of the National Cancer Institute 103 (2): 117–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignol, J. P. , Olivotto I., Rakovitch E., Gardner S., Sixel K., Beckham W., Vu T. T., Truong P., Ackerman I., and Paszat I.. 2008. “A Multicenter Randomized Trial of Breast Intensity‐Modulated Radiation Therapy to Reduce Acute Radiation Dermatitis.” Journal of Clinical Oncology 26 (13): 2085–92. [DOI] [PubMed] [Google Scholar]
- Presley, C. J. , Soulos P. R., Herrin J., Roberts K. B., Yu J. B., Killelea B., Lesnikoski B. A., Long J. B., and Gross C. P.. 2012. “Patterns of Use and Short‐Term Complications of Breast Brachytherapy in the National Medicare Population from 2008‐2009.” Journal of Clinical Oncology 30 (35): 4302–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, K. B. , Soulos P. R., Herrin J., Yu J. B., Long J. B., Dostaler E., and Gross C. P.. 2013. “The Adoption of New Adjuvant Radiation Therapy Modalities among Medicare Beneficiaries with Breast Cancer: Clinical Correlates and Cost Implications.” International Journal of Radiation Oncology Biology Physics 85 (5): 1186–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roof, K. , and Marks L. B.. 2014. “Breast Intensity Modulated Radiation Therapy versus Tissue Compensation: What's in a Name?” Practical Radiation Oncology 4 (1): 3–5. [DOI] [PubMed] [Google Scholar]
- Schnipper, L. E. , Smith T. J., Raghavan D., Blayney D. W., Ganz P. A., Mulvey T. M., and Wollins D. S.. 2012. “American Society of Clinical Oncology Identifies Five Key Opportunities to Improve Care and Reduce Costs: The Top Five List for Oncology.” Journal of Clinical Oncology 30 (14): 1715–24. [DOI] [PubMed] [Google Scholar]
- Sen, S. , Wang S. Y., Soulos P. R., Frick K. D., Long J. B., Roberts K. B., Yu J. B., Evans S. B., Chagpar A. B., and Gross C. P.. 2014. “Examining the Cost‐Effectiveness of Radiation Therapy among Older Women with Favorable‐Risk Breast Cancer.” Journal of the National Cancer Institute 106 (3): dju008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbie, J. W. , and Normand S. L.. 2008. “A Comparison of Methods for Combining Quality and Efficiency Performance Measures: Profiling the Value of Hospital Care Following Acute Myocardial Infarction.” Statistics in Medicine 27 (9): 1351–70. [DOI] [PubMed] [Google Scholar]
- Warren, J. L. , Yabroff K. R., Meekins A., Topor M., Lamont E. B., and Brown M. L.. 2008. “Evaluation of Trends in the Cost of Initial Cancer Treatment.” Journal of the National Cancer Institute 100 (12): 888–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, C. 2012. Health Status and Hospital Prices Key to Regional Variation in Private Health Are Spending. Research Brief.National Institute for Health Care Reform (NIHCR) under contract with Center for Studying Health System Change (HSC). [Google Scholar]
- Yabroff, K. R. , Lamont E. B., Mariotto A., Warren J. L., Topor M., Meekins A., and Brown M. L.. 2008. “Cost of Care for Elderly Cancer Patients in the United States.” Journal of the National Cancer Institute 100 (9): 630–41. [DOI] [PubMed] [Google Scholar]
- Yabroff, K. R. , Warren J. L., Schrag D., Mariotto A., Meekins A., Topor M., and Brown M. L.. 2009. “Comparison of Approaches for Estimating Incidence Costs of Care for Colorectal Cancer Patients.” Medical Care 47 (7 Suppl 1): S56–63. [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Baik S. H., Fendrick A. M., and Baicker K.. 2012. “Comparing Local and Regional Variation in Health Care Spending.” New England Journal of Medicine 367 (18): 1724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman, S. , Waidmann T., Berenson R., and Hadley J.. 2010. “Clarifying Sources of Geographic Differences in Medicare Spending.” New England Journal of Medicine 363 (1): 54–62. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix SA1: Author Matrix.
Appendix SA2: Current Procedural Technology (CPT) Codes and Healthcare Common Procedure Coding System (HCPCS) Codes Used to Identify Claims Corresponding to Surgery, Radiation Therapy, Systemic Therapy, Growth Factors, and Imaging Services.
Appendix SA3: Risk Factor Estimates for Total Medicare Expenditures for Cases and Controls (Expenditures Measured in $1,000s).


