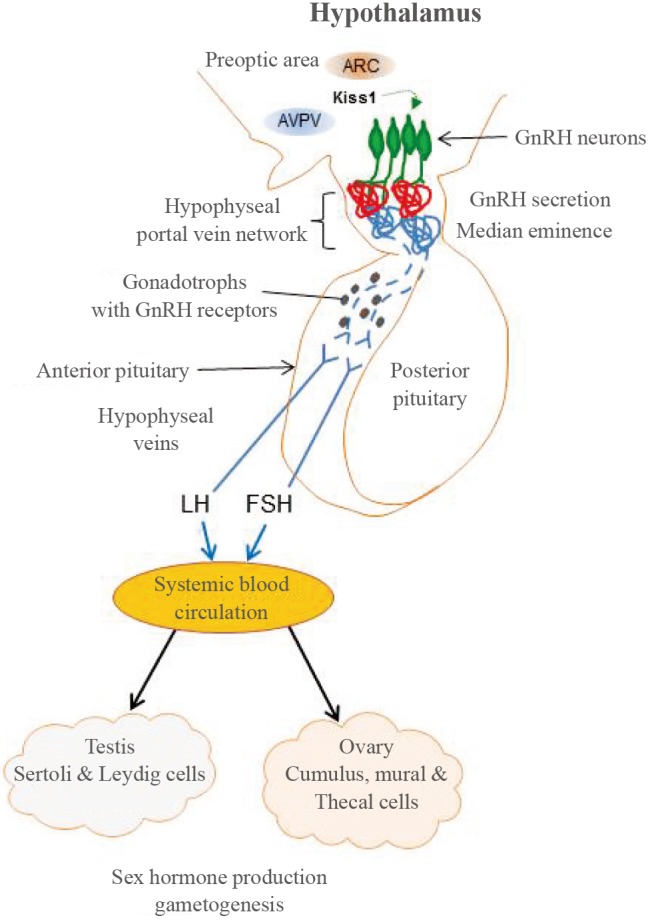
Fig. 1. The hypothalamic-pituitary-gonadal (HPG) axis. During early brain development, gonadotrophin-releasing hormone (GnRH)-releasing neurons (green) migrate from the nasal region to the hypothalamus, where they permanently reside and differentiate. Hypothalamic GnRH neurons secrete GnRH at the median eminence into the hypophyseal portal system and release pulsatile GnRH to the anterior pituitary. GnRH then binds to GnRH receptor 1 on the gonadotrophs to stimulate these cells to produce luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which enter the systemic blood stream through the hypophyseal veins. LH and FSH act on the gonads (Sertoli and Leydig cells in testes and cumulus, mural and thecal cells in ovaries) to induce steroidogenesis and germ cell production which, in turn, maintains sexual competence. The release of kisspeptin from the hypothalamic neurons located in the arcuate (ARC) and anteroventral periventricular nuclei within the preoptic area is critically important for the re-initiation of pulsatile GnRH secretion at puberty. The developmental failure or misregulation of any one or combination of the genes involved in GnRH migration, secretion, and activity at any stage of development may result in congenital hypogonadotropic hypogonadism and Kallmann syndrome. AVPV, anteroventral periventricular nucleus.