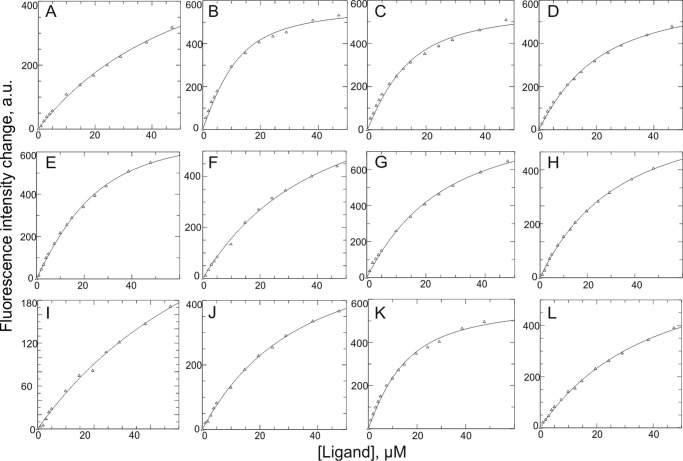
FIGURE 1.
Change in intrinsic fluorescence signal of SosCat variants upon addition of protein ligands, used to determine the dissociation constants Kd presented in Table 1. Individual panels show binding of WT SosCat with H-Ras·GDP (A), H-Ras·GTPγS (B), H-Ras·GppNp (C), H-Ras·GppCp (D), or H-RasY64A·GTPγS (E) and binding of SosCatW729E with H-Ras·GTPγS (F), SosHD-DH-PH-Cat with H-Ras·GTPγS (G), SosCatW729E with H-RasY64A·GTPγS (H), SosCatW729E with H-Ras·GDP (I), WT SosCat (preloaded with 30 μm H-RasY64A·GTPγS) with H-Ras·GTPγS (J), WT SosCat with K-Ras·GTPγS (K), and WT SosCat with K-RasG12V·GTPγS (L). The fluorescence data were fitted to a single site binding model using GraFit software (30). a.u., arbitrary units.