FIGURE 1.
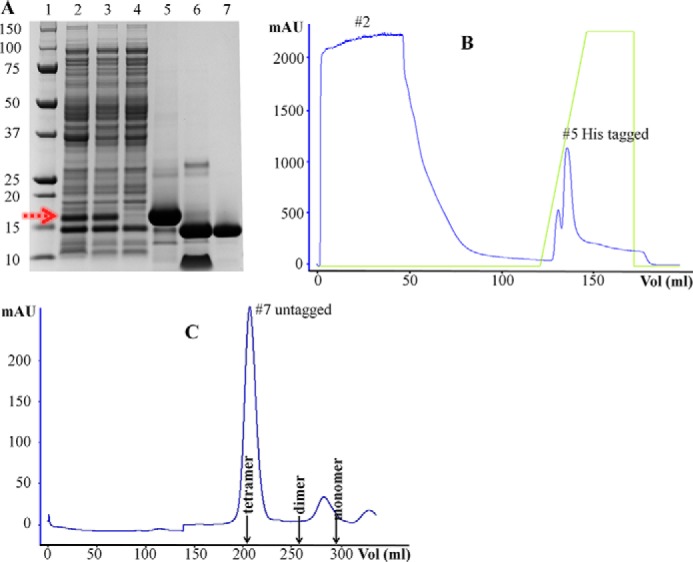
Purification of the SpPaaI by affinity and size exclusion chromatography yielding >95% pure protein. A, SDS-PAGE analysis of samples through the purification process: 1, size marker; 2, whole cell bacterial lysate showing over-expressed SpPaaI (red arrow); 3, soluble supernatant following centrifugation; 4, the flow-through of unbound proteins following loading of the supernatant onto the affinity column; 5, elution of His-tagged SpPaaI protein; 6, His tag removal from SpPaaI by tobacco etch virus protease; 7, SpPaaI following size exclusion chromatography. B, His tag affinity and C, size exclusion chromatography profiles with corresponding lane markers 2, 5, and 7 from A, showing a single, homogenous peak eluting from the size exclusion column at approximately molecular 60 kDa.
