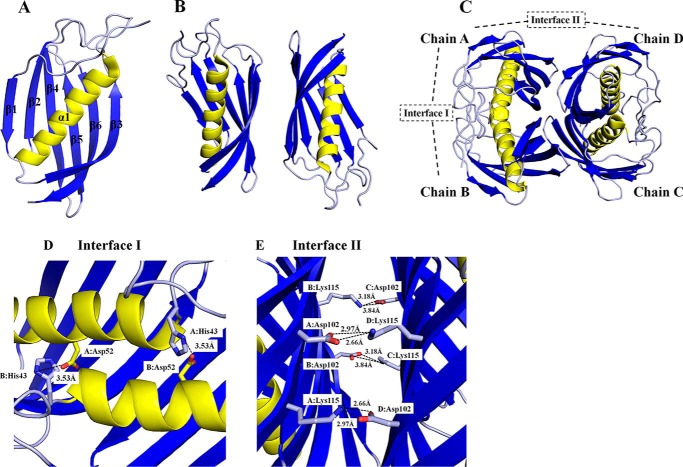
FIGURE 2.
Structure of SpPaaI. A, monomer of SpPaaI comprised a 6-stranded anti-parallel β sheet that cradles a central α-helix; B, two SpPaaI monomers in the asymmetric unit of the crystal; and C, quaternary structure and biological unit comprising a dimer of double hotdog domains orientated back to back with respect to the central α-helices. The salt bridge interactions within the interfaces are shown for D, interface I involving interactions between His43 and Asp52 within the double hotdog dimer (chain A:B and C:D); and E, interface II salt bridge interactions between Asp102 and Lys115 within the biological tetramer (chains A:D and chains B:C).