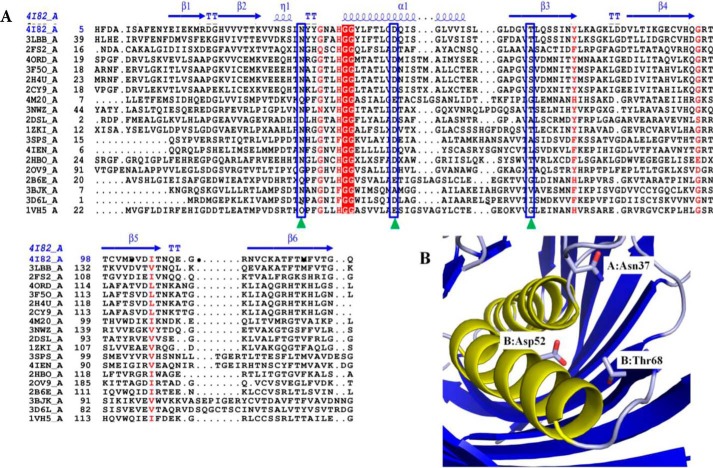
FIGURE 6.
Structural alignment of SpPaaI. A, primary and secondary structure alignment of SpPaaI using the ENDscript/ESPript web server: helices are represented by α, strands by β, and turns with T. The predicated active site amino acid residues are indicated by green triangles and blue boxes. Two highly conserved Gly residues that precede the central helix are highlighted in red. B, active site residues of SpPaaI are contributed from residues on both chains of the dimer: Asn37 of chain A and Asp52 and Thr68 of chain B in dimer AB are shown.