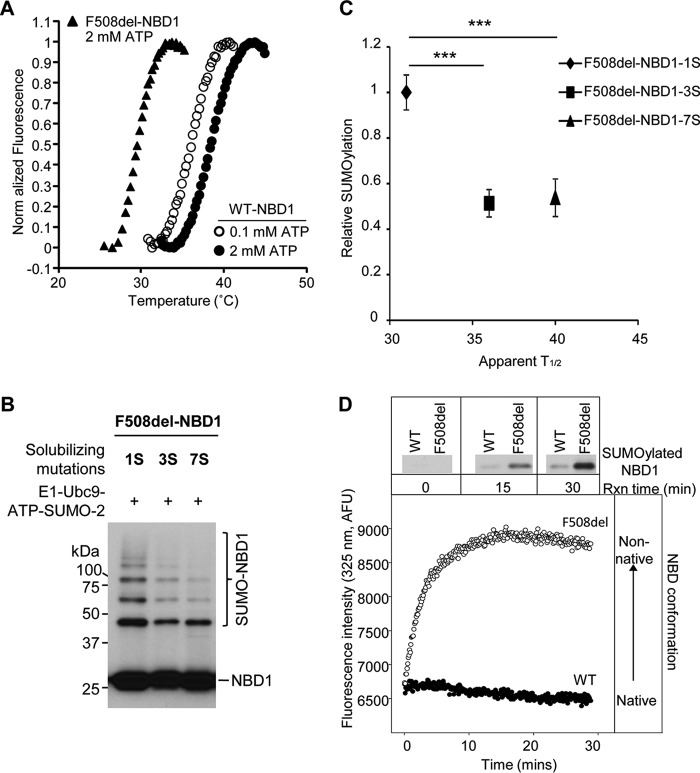
FIGURE 7.
SUMOylation is inversely proportional to NBD1 thermal stability. A, temperature-dependent unfolding of NBD1. Purified WT and F508del 1S-NBDs were diluted into buffers, and the fluorescence of SYPRO orange, which interacts with exposed hydrophobic regions, was monitored. The apparent T½ values for the unfolding transitions of WT NBD1 at 2 mm ATP (closed circles) and 0.1 mm ATP (open circles) and for F508del NBD1 at 2 mm ATP (triangles) are provided in the text and in C and are in agreement with previously determined values (25). B, solubilizing and revertant mutations (25) reduce the extent of F508del NBD1 SUMOylation in vitro. C, relation between relative in vitro SUMOylation (Fig. 7B) and thermal stability data for 1S, 3S, and 7S F508del NBD1s (25) (***, p = 0.0003, 3S; p = 0.0002, 7S). D, SUMOylation follows the kinetics of NBD1 transition to a non-native conformation. WT and F508del NBD1 SUMO modification (upper panels) were determined at 0.1 mm ATP and 27 °C at the times indicated (data from Fig. 6A). Immunoblots were performed using NBD1 (660) antibody. Intrinsic fluorescence values at 325 nm were normalized to the values at 5 min for WT and F508del 1S-NBD1.