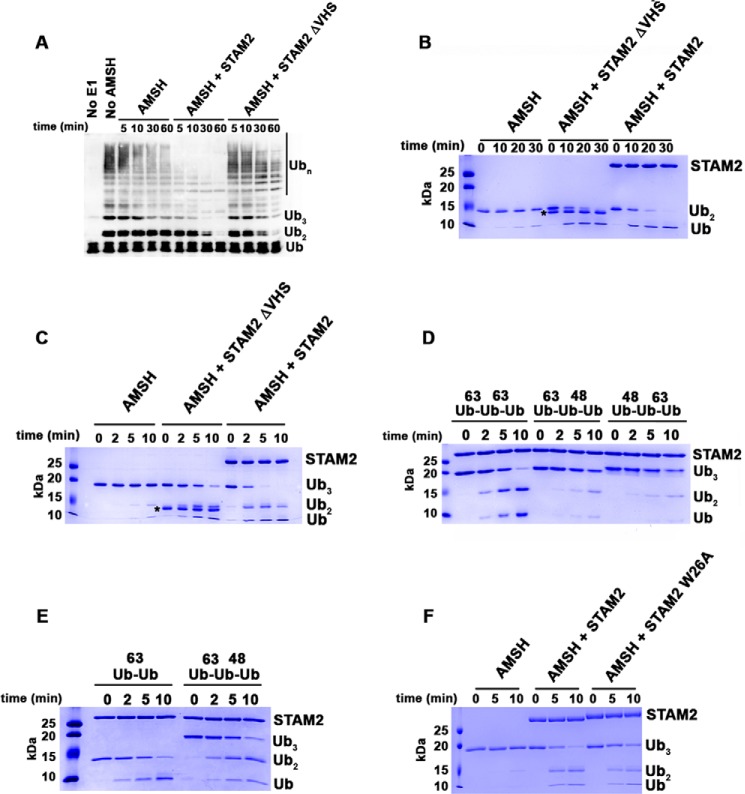
FIGURE 5.
The VHS domain of STAM2 directs AMSH to cleave longer Lys63-linked ubiquitin chains. A, cleavage of Lys63-linked ubiquitin chains by AMSH (50 nm) at 37 °C in the absence of STAM2 or in the presence of 5 μm STAM2 possessing and missing the VHS domain. Detection of ubiquitin chains was done by anti-Ub Western blot analysis. B, cleavage of Lys63-linked di-Ub (4 μm) by AMSH (50 nm) at 37 °C in the absence of STAM2 or in the presence of STAM2 (5 μm) possessing and missing the VHS domain. The asterisk denotes the position of bands corresponding to STAM2ΔVHS. C, similar to B, but tri-Ub is the substrate. D, cleavage of Lys63-linked tri-Ub chains or mixed tri-Ub chains (4 μm) by AMSH (50 nm) in the presence of STAM2 (5 μm) at room temperature. E, cleavage of mixed tri-Ub compared with Lys63-linked di-Ub. The experiment was performed as described in D. F, cleavage of Lys63-linked tri-Ub by AMSH in the absence of STAM2 or in the presence of STAM2 WT or mutant (W26A). The experiment was performed as described in B.