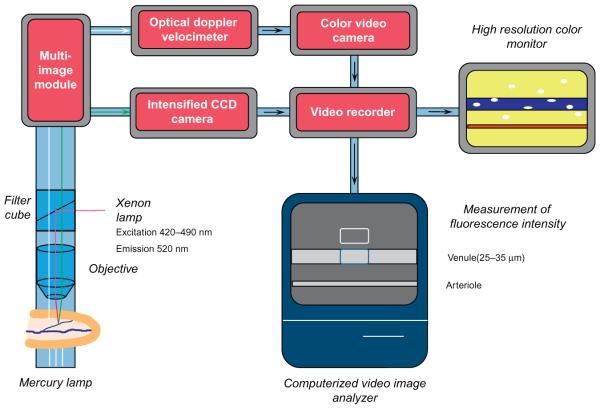
Figure 2.
Graphical representation of components required for intravital microscopic observation of the living microcirculation by trans- or epi-illumination. For transillumination, light from a mercury light source is directed through exteriorized thin (<100 mm) tissues to a salt water immersion microscope objective that focuses the collected image onto a color video camera. Images from the camera are recorded by a DVD recorder and presented on a color television monitor. An optical Doppler velocimeter is incorporated into the light path between the microscope objective and video camera to monitor erythrocyte velocity. Computerized fluorescence intravital microscopy can also be incorporated into this system to quantify venular protein leakage, chemokine or adhesion molecule expression, or oxidant production. Using epifluorescence microscopy, light from a xenon source directed to the tissue under observation via a filter cube, which excites the fluorochrome of interest, causing it to emit light that is collected via the objective and directed to an intensified CCD camera, captured onto a computer (or to videotape or video disc by use of a video or digital video recorder, respectively) for computerized image analysis of fluorescence intensity. A multi-image module directs light to either the color video camera or the CCD camera. Vessel diameter is measured using a videocaliper (not shown) and the numbers of rolling and adherent leukocytes, leukocyte rolling velocity, and mean red cell velocity a quantified during off-line analysis of the collected images. Not shown for simplicity is the animal whose tissues are being observed.