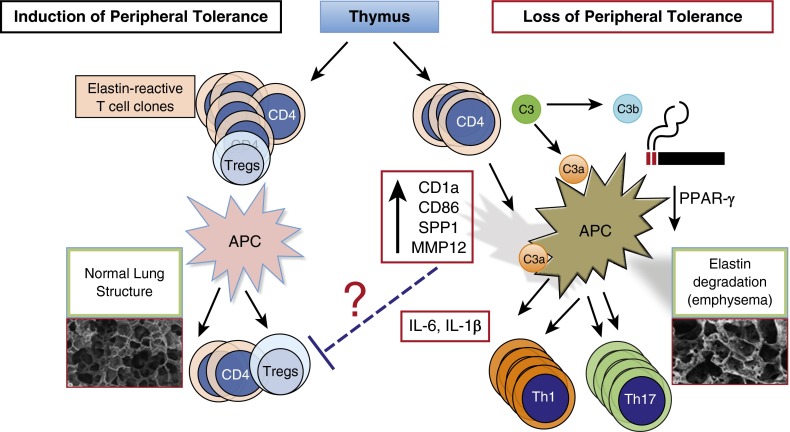
Figure 1.
Schematic diagram of possible mechanisms involved in loss of peripheral tolerance in cigarette smoke–induced emphysema. Under normal conditions, signals from tolerogenic antigen-presenting cells (APCs) in the lungs or regional lymph nodes promote maintenance of peripheral tolerance; self-reactive T cells emerging from the thymus remain nonresponsive to self-antigens (left side). However, cigarette smoke downregulates peroxisome proliferator-activated receptor (PPAR)-γ, which in turn activates SPP1 gene expression and increases several costimulatory molecules (e.g., CD1a, CD86, major histocompatibility complex class II) on lung APCs. In susceptible smokers, possibly with lower thresholds for developing autoimmune T cells (e.g., higher number of self-reactive T cells or exaggerated responses to cigarette smoke activation of APCs), antigen-specific autoreactive helper T Type 1 (Th1) and Th17 cells clonally expand when they come in contact with activated APCs and promote a slow but progressive form of lung destruction (right side). Cigarette smoke induces recruitment of innate immune cells. Enzymes released by activated neutrophils and macrophages (e.g., neutrophil elastase, matrix metalloproteinase 12 [MMP12]) could activate complement proteins (e.g., C3, C3a) and their receptors (e.g., C3aR), thereby accelerating the development of autoreactive Th1 and Th17 cells and lung destruction in smokers. Tregs = regulatory T cells.