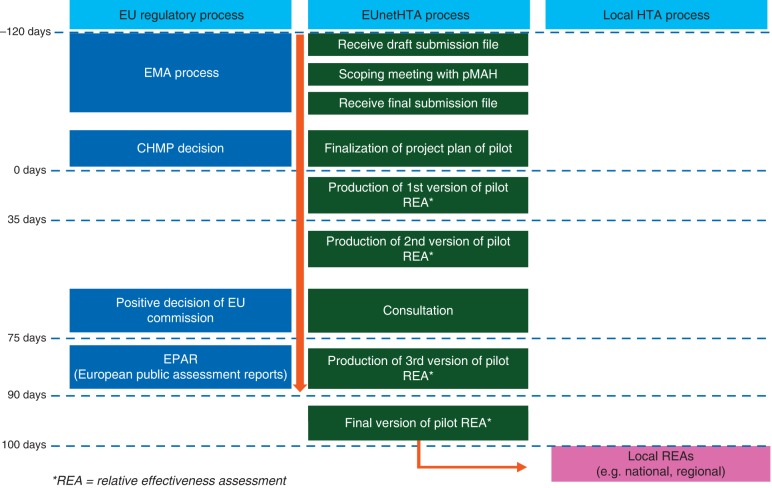
Figure 2.
EUnetHTA project of parallel assessment of regulatory bodies and HTA agencies (adapted from http://www.eunethta.eu). The aim of this project is to have early parallel assessments on new drugs between the regulatory body (EMA) and HTA agencies including early consultations. The participation of the HTA agencies is voluntary and the HTA bodies are selected by the sponsor.