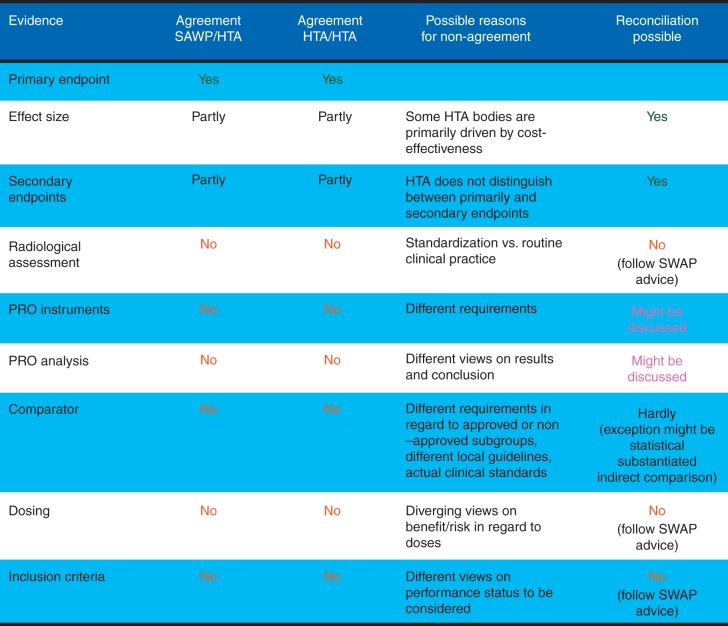
Figure 3.
EMA - HTA parallel scientific advice. Eventiary requirements in an oncologic case study (adapted from Britta Paschen, EMA/HTA workshop 2013; http://www.ema.europa.eu/docs/en_GB/document_library/Presentation/2013/11/WC500155673.pdf) (PRO = patient reported outcome; SAWP = Scientific advice working party; HTA = Health technology assessment).