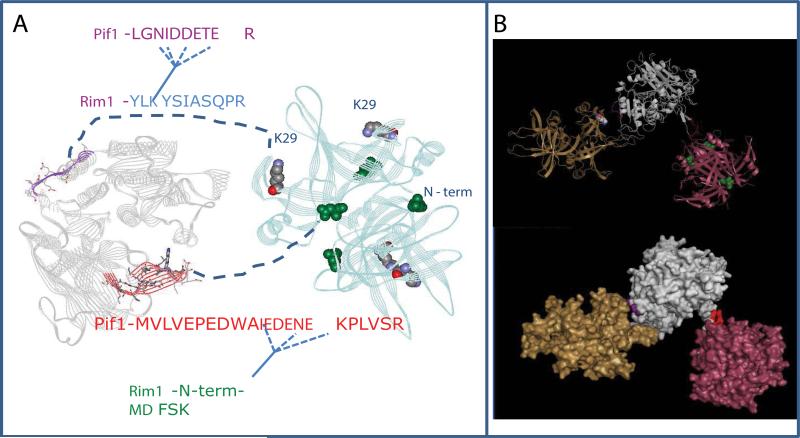
Figure 6. Model of Pif1-Rim1 interaction.
A, Summary of the protein-protein interactions defined in cross-linking experiments. The model of Pif1 helicase domain is on the left. The X-ray structure of Rim1 tetramer is on the right. The two interaction sites identified in this work are highlighted. The X-ray structure has N-terminal aspartate instead of methionine. We propose the model of Rim1-Pif1 interaction, where 1) the beta-hairpin of Pif1, highlighted in red fits into the cleft between two Rim1 dimers; and 2) the stretch of acidic residues within Pif1's peptide LGNIDDETER is likely to be in the vicinity of K29 residue of Rim1. B, The possible spatial arrangement of Rim1-Pif1 interactions. It is unlikely that the two interaction sites are realized at the same time within the (Rim1)4:Pif1 heterodimer. Instead, the most consistent with the current structural information is the model, where two Rim1 tetramers participate in the interaction with Pif1. Pif1 helicase domain is shown in grey, Rim1 tetramers are shown in yellow and pink.