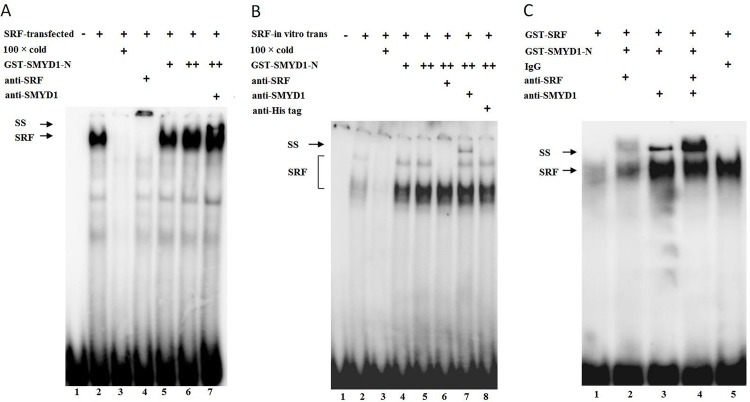
Fig 6. SMYD1 forms complex with SRF and enhances SRF DNA binding activity.
Nuclear protein was isolated from 293T cells. (A) EMSA was performed using purified GST-SMYD1-N fusion protein, SRF- in vitro trans-protein and 32P-labeled oligonucleotide probes containing a consensus binding motif for SRF. Lane 1 was vector control, lane 3 contained 100× cold as a competitor. SMYD1 dose-dependently enhances SRF DNA binding activity (lanes 2, 5, 6). The anti-SRF antibody (lane 4) and anti-SMYD1 antibody (lane 7) were used for the supershift assay. (B) EMSA was performed using purified GST-SMYD1-N fusion protein, SRF-transfected-protein and 32P-labeled oligonucleotide probes containing a consensus binding motif for SRF. Lane 1 was vector control, lane 3 contained 100× cold as a competitor. SMYD1 dose-dependently enhances SRF DNA binding activity (lanes 4, 5). The anti-SRF antibody (lane 6) and anti-SMYD1 antibody (lane 7) were used for the supershift assay. The anti-His antibody was used as a control (lane 8). (C) EMSA was performed using purified GST-SMYD1-N fusion protein, SRF fusion protein and 32P-labeled oligonucleotide probes containing a consensus binding motif for SRF. The anti-SRF antibody (lane 2, 4) and anti-SMYD1 antibody (lane 3) were used for the supershift assay. The IgG was used as a control (lane 5).