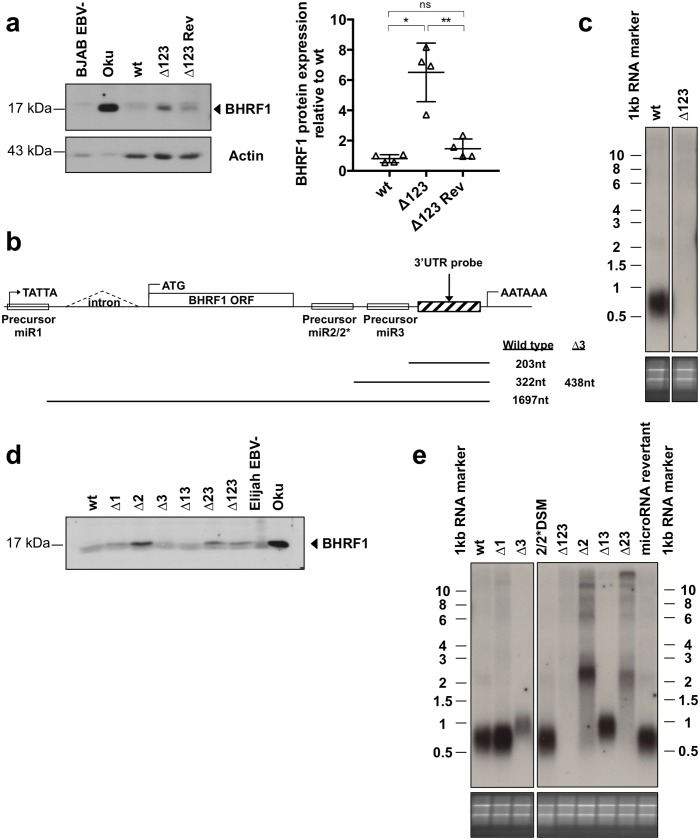
Fig 6. miR-BHRF1-2 downregulates the BHRF1 protein in established LCLs.
(a) Western blot with a BHRF1-specific antibody performed on LCLs established from a primary B-cell sample infected with B95-8 wt, Δ123 or the Δ123 revertant virus (Δ123 Rev) virus and tested 2 months after infection. Actin served as a loading control. The EBV negative BJAB BL and the Wp-restricted Oku-BL served as negative and positive control for BHRF1 protein expression, respectively. The right panel shows the ImageJ-quantified BHRF1 protein expression in four donors 2 months after infection, normalized for differences in actin expression. (b) This schematic describes the BHRF1 locus and gives the length of the different BHRF1 RNA species that would arise from Drosha-mediated processing of the different miRNAs. The sizes are given exclusive of the polyA tails. (c) 1.5μg polyA+ RNA extracted from wild type- or Δ123-transformed LCLs was analyzed by northern blotting 45 days after infection. The blot was hybridized with a probe specific for the 3’UTR of BHRF1 downstream of miR-BHRF1-3, shown in (c). The bottom panel shows the agarose gel stained with ethidium bromide. (d) BHRF1 protein expression in LCLs 45 days after infection with a panel of viruses lacking one or several BHRF1 miRNAs was determined by western blotting. (e) 1.5μg polyA+ RNA was isolated from LCLs generated from one primary B-cell sample by infection with B95-8 wild type or the different single and double miR-BHRF1 knockout viruses. The northern blot was hybridized with a probe specific to the 3’UTR of BHRF1 downstream of miR-BHRF1-3, as depicted in (c).