Abstract
Bone is the most common site for metastasis in patients with solid tumours. Bisphosphonates are an effective treatment for preventing skeletal related events and preserving quality of life in these patients. Zoledronic acid (ZA) is the most potent osteoclast inhibitor and is licensed for the treatment of bone metastases. Clodronate and pamidronate are also licensed for this indication.
In addition, ZA has been demonstrated to exhibit antitumour effect. Direct and indirect mechanisms of anti-tumour effect have been postulated and at many times proven. Evidence exists that ZA antitumour effect is mediated through inhibition of tumour cells proliferation, induction of apoptosis, synergistic/additive to inhibitory effect of cytotoxic agents, inhibition of angiogenesis, decrease tumour cells adhesion to bone, decrease tumour cells invasion and migration, disorganization of cell cytoskeleton and activation of specific cellular antitumour immune response. There is also clinical evidence from clinical trials that ZA improved long term survival outcome in cancer patients with and without bone metastases. In this review we highlight the preclinical and clinical studies investigating the antitumour effect of bisphosphonates with particular reference to ZA.
Keywords: Zoledronic acid, Bisphosphonates, Apoptosis, T-cells, Angiogenesis, Antitumour
1. Introduction
Bisphosphonates are proven to be effective in preventing/delaying skeletal-related events in patients with bone metastases and potentially preserving functional independence and quality of life. This effect is mediated by the inhibitory effect of bisphosphonates on osteoclasts.
Recently, it has been reported that bisphosphonates may have anti-tumour effect as well.
There are two classes of bisphosphonates that differ with regard to structure and mechanism of action [1]. The first one includes pyrophosphate-resembling bisphosphonates, such as clodronate and etidronate, which are metabolically incorporated into nonhydrolyzable adenospine tri-phosphate (ATP) analogues that act as inhibitors of ATP-dependent enzymes. The second class which is more recent and potent includes nitrogen-containing bisphosphonates (N-BPs), such as alendronate, pamidronate, risedronate, ibandronate and zoledronic acid (ZA).
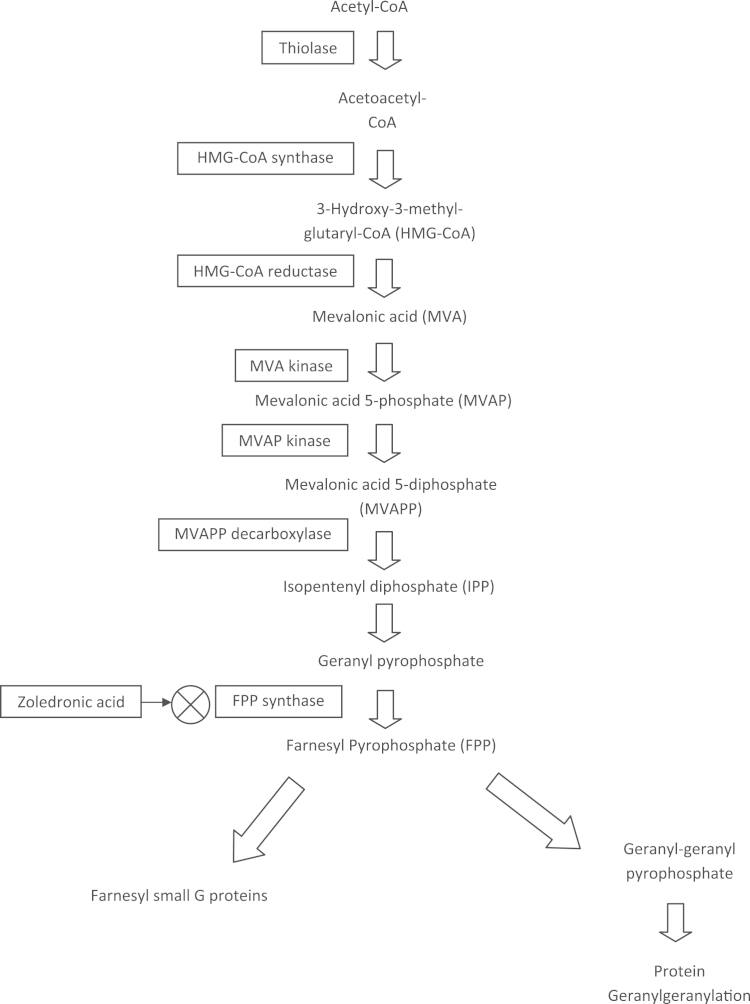
N-BPs inhibit a key enzyme, farnesyl diphosphonate (FPP) synthase, in the biosynthetic mevalonate pathway. As a result, these compounds interfere with a variety of cellular functions essential for the bone-resorbing activity and survival of osteoclasts. Several intermediates in this pathway (Fig. 1), including farnesyl pyrophosphate and geranylgeranyl pyrophosphate, are required for the post-translational modification (i.e., prenylation) of guanosine triphosphate-binding proteins such as Ras, Rho, and Rac. These signalling molecules are involved in the regulation of cell proliferation, cell survival, and cytoskeletal organization [2], [3].
Fig. 1.
Flowchart showing the mevalonate pathway.
ZA is reported to be more potent inhibitor of farnesyl diphosphate synthase than the other bisphosphonates risedronate, ibandronate, incadronate, alendronate, and pamidronate [4].
Preclinical findings provide insight into possible mechanisms of action of bisphosphonates that may explain their ability to inhibit tumour cells. This report reviews the preclinical and clinical data investigating the anti-tumour effects of ZA.
1.1. Preclinical rationale for potential anticancer effects of ZA
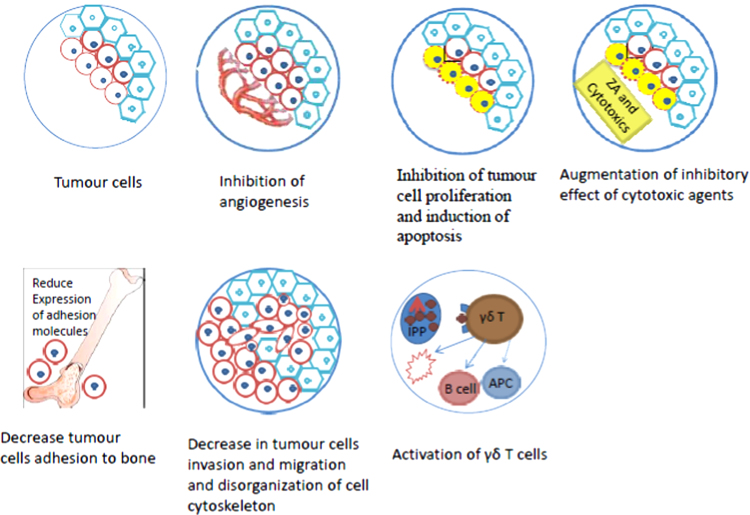
Preclinical data indicate that possible anti-cancer mechanisms of ZA (and other bisphosphonates) may include (Fig. 2):
-
•
Inhibition of tumour cell proliferation and induction of apoptosis.
-
•
Augmentation of inhibitory effect of cytotoxic agents (additive and synergistic effect).
-
•
Inhibition of angiogenesis.
-
•
Decrease in tumour cell adhesion to bone.
-
•
Decrease in tumour cells invasion and migration and disorganization of cell cytoskeleton.
-
•
Activation of γδ T cells.
-
•
Effects on tumour macrophage infiltration.
Fig. 2.
Possible mechanisms of anti-tumour effect of ZA.
Preclinical studies investigating these possible mechanisms of action are presented below and summarized in Table 1.
Table 1.
Summary of pre-clinical and early clinical [52], [56], [57] studies and publications describing various mechanisms of anti-tumour activity of nitrogen-containing bisphosphonates.
| Reference number | Bisphosphosphonate(s) used | Concentrations/doses studied | Cell lines | Results |
|---|---|---|---|---|
| Proliferation inhibition and apoptosis induction | ||||
| [6] | ZA | Up to 10 µM | Fibrosarcoma | Inhibition, cell cycle arrest |
| [7] | ZA | 80 µg/kg | Osteosarcoma | Inhibition of growth |
| [8] | ZA | 1.55–4.48 µg/ml | Giant cell tumour, myeloma, renal cell carcinoma | Dose dependent cytotoxicity |
| [9] | ZA | 10–100 µM | Oral carcinoma: 4 cell lines | Induction of apoptosis (concentration dependent) |
| [10] | ZA | 6.25–100 µM | Renal cell carcinoma: 3 cell lines | Induction of apoptosis |
| [11] | ZA | Up to 20 µM | Multiple myeloma | Induction of apoptosis |
| [13] | ZA | Up to 10 µM | Prostate and breast cancer | Induction of apoptosis (concentration dependent) |
| [12] | ZA | 100 µM | Non-small cell lung cancer (NSCLC) | Cell cycle arrest, apoptosis induction |
| Additive and synergistic effect with cytotoxic agents | ||||
| [14], [15] | ZA | 12.5–50 µM | Hormone resistant prostate cancer | Additive and synergistic effect with docetaxel |
| 5–30 µM | ||||
| [16] | ZA | 10–100 µM | Breast cancer | Additive effect with paclitaxel |
| [17] | ZA | 100 µM | NSCLC | Additive effect with cisplatin |
| [6] | ZA | Up to 10 µM | Fibrosarcoma | Additive effect with various chemotherapy agents |
| [18] | ZA | 100 µg/kg | Breast cancer | Additive effect with doxorubicin (when used with or after doxorubicin) |
| [19] | ZA | Up to 25 µM | Breast cancer | Synergistic effect with letrozole |
| Inhibition of angiogenesis | ||||
| [12] | ZA | 10–100 µM | Non-small cell lung cancer | Reduction of VEGF secretion |
| [28] | ZA | Up to 200 µM | Endothelial progenitor cells | Reduction of viable cells |
| Decrease in tumour cell adhesion to bone | ||||
| [37] | ZA | 100–1000 µM | Multiple myeloma | Decrease in bone marrow stromal cells, decreased expression of adhesion molecules |
| [39] | Ibandronate | 1000 nM | Breast and prostate cancer | Inhibition of tumour cell binding to bone matrices |
| [40] | Clodronate, pamidronate, olpedronate, etidronate and ibandronate | 1–100 µM | ||
| [38] | Ibandronate | 5 picoM | ||
| Pamidronate | 0.1 µM | |||
| Clodronate | 100 µM | |||
| Inhibition of tumour cell invasion and migration | ||||
| [41], [43] | Reviews | Breast and prostate cancer | Inhibition of visceral metastasis | |
| [42] | ZA | 1 µM | ||
| [44] | ZA | 0.5–5 µmg/mouse | ||
| [45] | Alendronate | 30 µM | Ovarian cancer | Inhibition of cell migration |
| Activation of γδ T cells | ||||
| [51] | Alendronate, ibandronate and pamidronate | 0.9–4 µM | Multiple myeloma | Reduced plasma cell survival due to activation of γδ T cells |
| [52] | ZA | 4 mg every 3 weeks (4 doses) | Metastatic bone disease | Maturation of γδ T cells (in vivo) |
| [53] | ZA | 1 µM (with IL-2) | Multiple myeloma | Increase in number of γδ T cells |
| [55] | ZA | 1 µM (with IL-2) | Small cell lung cancer and fibrosarcoma | Marked increase in sensitivity to lysis by γδ T cells |
| [54] | ZA | 3 µM | Pancreatic cancer | Increased cancer cell cytotoxicity by γδ T cells |
| [56] | ZA | Unknown | Prostate cancer | Activation and increase in number of γδ T cells |
| [57] | ZA | 4 mg (1 dose) | Breast cancer | Activation of γδ T cells |
ZA=zoledronic acid; µM=micromolar; mg=milligrams; picoM=picomolar; γδ=gamma delta.
1.1.1. Inhibition of tumour cell proliferation and induction of apoptosis
ZA inhibits a key enzyme of the mevalonate pathway, farnesyl diphosphonate synthase.
Inhibition of this enzyme prohibits formation of isoprenoids, such as farnesyl diphosphate (FPP) and geranylgeranyl diphosphate (GGPP), which are required for regular prenylation of small GTPbinding proteins, like Rho and Ras (Fig. 1) [5].
There is significant preclinical evidence to support the direct antitumour effect of ZA. In a preclinical study, ZA strongly inhibited in vitro proliferation, arrested cell cycle between S and G2/M phases, and induced the apoptosis of human fibrosarcoma cells [6]. The same group of investigators reported inhibition of growth of osteosarcoma cells at the primary and secondary sites in a murine model [7].
In another study, Zwolak et al. showed that ZA can be released from bone cement (formed with increasing concentrations of ZA, up to 1 mg/1.5 cm3 of bone cement) and the proliferation assay showed ZA to have significant dose dependent cytotoxicity in cultures of stromal giant cell tumour, multiple myeloma, and renal cell carcinoma cells [8]. In a separate study, ZA showed direct antitumor effects against four oral carcinoma cell lines at concentrations ranging from 10 to 100 µM. ZA activated the potent pro-apoptotic pathways caspase-3, -8 and -9 and induced cellular apoptosis and increased the number of cells in apoptosis. Western blot analysis showed that ZA increased cleaved anti-human poly(ADP-ribose) polymerase expression and decreased Bcl-2 and Bid expression [9]. In renal cancer cell lines (ACHN, A-498 and CAKI-2), a significant reduction in viable cells was seen for all three cell lines following treatment with ZA (at concentrations of 6.25–100 µM), compared with untreated controls. A concomitant increase in the apoptosis significant caspase-dependent M30 antigen was demonstrated. This effect could be blocked by the pan-caspase inhibitor Z-VAD [10]. Similar apoptotic effect of ZA (in concentrations of 5–40 µM) was seen in dexamethasone resistant multiple myeloma (MM) cells [11].
The preclinical evidence of antitumour effect is well documented for other solid tumour models as lung, breast and prostate [12], [13].
It seems that at least in the preclinical setting, there is substantial evidence supporting the anti-proliferative effect of ZA mainly by inducing apoptosis. This mechanism is amenable to be studied in clinical setting. Although a 15 min intravenous infusion of 4 mg ZA achieves a maximum concentration 0.97 µM (Product Information: RECLAST(R) IV injection, zoledronic acid IV injection. Novartis Pharma Stein AG, Stein, Switzerland, 2008), some preclinical experiments in mouse models with metastatic breast cancer to bone have demonstrated in-vivo that alternative dosage regimens with ZA (daily and weekly administration in divided doses) can cause decrease in tumour burden in the bone (PMID: 17312309). Therefore daily or weekly treatment regimens of ZA (in divided doses) may be amenable to further investigation in clinical trials.
1.1.2. Augmentation of inhibitory effect of cytotoxic agents (additive and synergistic effect)
Enhancing the cytotoxic effect of anti-cancer drugs on tumour cells has always been an active area of research. In the clinical setting, cancer patients on systemic anti-cancer therapy (chemotherapy or hormonal therapy) may receive ZA for reasons other than its possible anti-tumour effect. It seems attractive to investigate potential additive/synergistic effect of ZA to chemotherapy and/or hormonal therapy. The available literature confirms this effect in pre-clinical studies.
In one study, hormone-refractory prostate cancer cell lines (PC3 and DU145) were treated with increasing concentrations of ZA in the absence or presence of docetaxel. After 72 h incubation, ZA at concentration of 25 μM reduced the viable cell number to 68% and 98% for PC3 and DU145 cells respectively. Docetaxel, on the other hand, at a concentration of 0.1 ng/ml, had no effect on the viability. However, a combination of ZA and docetaxel reduced the cell number to 60% and 81% respectively. ZA in the concentration range 12.0–50 μM enhanced the antitumoral effects of docetaxel (0.01–1 ng/ml) in an additive and/or synergistic manner for both cell lines [14].
These results were repeated by another group of investigators using the same agents and cell lines [15]. Additive/synergistic inhibitory effect of ZA is also documented with other chemotherapy agents as paclitaxel, docetaxel, doxorubicin, etoposide, 5-fluorouracil, gemcitabine, and cisplatin [6], [16], [17].
The additive and/or synergistic effect of ZA may be dependent on the sequence of treatment. Studies suggest that Sequential treatment with chemotherapy followed by ZA elicit substantial antitumor effects compared to the reverse sequence. Female MF1 nude mice were inoculated human breast cancer MDA-MB-436 cells. On day 7, the mice were injected weekly for 6 weeks with saline, doxorubicin or ZA alone, combination of both, ZA followed 24 h later by doxorubicin, or doxorubicin followed 24 h later by ZA. Treatment with doxorubicin followed by ZA almost completely abolished tumour growth. Tumours from mice in this group had significantly more caspase-3-positive cells than tumours from mice treated with saline, with ZA alone or with ZA followed by doxorubicin. This increase in the number of caspase-3-positive cells was mirrored by a decrease in the number of tumour cells positive for the proliferation marker Ki-67. The authors concluded that sequential treatment with doxorubicin followed by ZA elicited substantial antitumor effect [18].
Synergism is not only limited to chemotherapy. There is evidence that ZA can augment inhibitory effect of hormonal therapy. Aromatase-expressing breast cancer cells were treated with letrozole (100 nM) and ZA(10 μM) either simultaneously or in sequence, each for 24 h. Letrozole and ZA induce levels of apoptosis in breast cancer cells in vitro that are significantly greater compared with treatment with each drug alone. However, this potentially synergistic relationship was drug-sequence dependent, occurring only when cells were treated with letrozole, followed by ZA [19].
These studies suggest the additive and synergistic effect of ZA with other anti-cancer agents. The exact mechanisms remain unknown but down-regulation of anti-apoptotic genes has been suggested [15]. Preclinical data showed increased anti-tumour efficacy if ZA is sequenced after chemotherapy compared to the opposite sequence [20]. Based on this observation, most clinical trials investigating the role of ZA in the neo-adjuvant setting including NEOZOTAC [21], ANZAC [22] and NeoAZURE [23] administered ZA after chemotherapy. The adjuvant phase III AZURE study is also an example where investigators used ZA after chemotherapy [24].
The sequence of administration may need to be addressed in future clinical trials. One clinical investigational approach will be to randomize patients in the neoadjuvant or metastatic setting to different sequences of ZA and specific anti-cancer therapy. Pre-treatment and post-treatment biopsies to study molecular changes may help elucidate mechanisms of this additive/synergistic effect.
1.1.3. Inhibition of angiogenesis
Angiogenesis is a prerequisite for the progressive growth of solid tumours and their metastasis [25]. In malignant tumours, the development of new vessels is directed and regulated by a complex network of endogenous pro-angiogenic factors, e.g. vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) and by factors secreted by the tumour itself. Angiogenesis enables the tumour to metastasize to various sites. Thus, inhibition of angiogenesis is a promising strategy in the treatment of malignant tumours.
There is evidence that ZA inhibits differentiation of cultured human peripheral blood mononuclear cells into endothelial progenitor cells [26]. ZA inhibits proliferation of human endothelial cells in vitro and reduces vessel sprouting in cultured aortic rings and chicken egg chorioallantoic membrane assay [27]. This study also provided the first in-vivo evidence that ZA affects angiogenesis occurring in non-mineralized tissue. However, ZA dose of 100 μg/kg was required to achieve any significant antiangiogenic effect.
In one study, ZA at concentration of 10–100 μm induced dose dependent reduction both of mRNA and protein expression of VEGF in A549 NSCLC cells associated with parallel decrease in VEGF secretion in the culture medium after 2 days of treatment [12].
In another study, the viability of human umbilicord vein endothelial cells (HUVEC) and endothelial progenitor cells (EPC) was significantly reduced after incubation with bisphosphonates compared with the non-treated control groups. The nitrogen-containing bisphosphonates pamidronate and ZA had the greatest impact on the cells, whereas the inhibitory effect of clodronate and ibandronate on these cells was less distinct [28].
An in vivo studies show that ZA (20 μg/mouse) for 3 times a week for3 consecutive weeks inhibited angiogenesis in Matrigel plugs and inhibited the growth and neo-angiogenesis of CG5 xenografts in athymic nude mice [29]. Another in vivo study documented that capillary densities were significantly lower in mice pre-treated with low dose (30 μg/kg) and high dose ZA (100 μg/k) than in control mice in response to surgically induced hypoxia. Ischaemic tissue from ZA pre-treated mice also showed impaired mobilization of endothelial progenitor-like cells and lower levels of the active form of MMP-9 and VEGF compared to ischaemic tissue from control mice [30].
Another in-vivo supporting evidence that ZA uptake occurs in non-mineralized tissue is provided by Stresing et al. ZA inhibited the revascularization of the prostate gland in testosterone-stimulated castrated rats. It also induced intracellular accumulation of isopentenyl pyrophosphate (IPP) in endothelial cells by blocking the activity of the IPP-consuming enzyme FPPS. Thus, these results indicated that N-BPs inhibited angiogenesis in a FPPS-dependent manner [31].
The more potent antiangiogenic effect of nitrogen-containing bisphosphonates compared to other bisphosphopnates in preclinical studies mirrors their superior efficacy seen in clinical setting.
Recent in-vivo evidence showed that bone marrow endothelial progenitor cells (BM EPCs) are affected after ZA administration. On flow cytometry, BM EPCs increased in response to acute parathyroid hormone therapy but not when treatment was combined with ZA. This observation provides indirect suggestion to uptake of ZA by bone endothelial cells [32].
Accumulating reports have shown that cancer patients who have received nitrogen-containing bisphosphonates such as ZA occasionally manifest bisphosphonate-related osteonecrosis of the jaw (BRONJ) following dental treatments including tooth extraction. Little is known about the pathogenesis of BRONJ to date. The results of Kobayashi Y and his colleagues′ work showed that ZA alters oral bacterial behaviours and delays wound healing of the tooth extraction socket by inhibiting osteogenesis and angiogenesis. They conclude that these actions of ZA may be relevant to the pathogenesis of ONJ [33]. This conclusion is supported by clinical observation of higher incidence of ONJ in cancer patients treated with ZA and anti-angiogenic agents [34], [35].
The available data not only demonstrate the importance of anti-angiogenic properties of ZA but also elucidates the potential mechanism and molecular targets of this property.
Most of preclinical supporting evidence for the anti-angiogenic properties of ZA comes from in vitro models and therefore further evaluation in more animal models and humans is needed. One challenge in clinical studies will be the need for higher concentrations which seem to be needed to achieve anti-angiogenesis.
1.1.4. Decrease in tumour cell adhesion to bone
Recent studies have shown the importance of the microenvironment in the pathophysiology of metastatic bone disease. Adhesive interactions of tumour cells play an important role in up-regulating the production of cytokines and growth factors by bone marrow stromal cells, which consequently enhance tumour growth, bone destruction, and tumour survival [36]. Multiple myeloma (MM) tumour cells naturally home at bone marrow (BM) and provide a model for studying the effect of bisphosphonates on behaviour of tumour cells in this environment. Adhesion molecules facilitate binding of MM cells to the BM stroma. There is rising laboratory evidence that ZA decrease tumour cells adhesion to bone microenvironment.
In one study, BM stromal cells, obtained from BM mononucleated cells of eight patients with MM, were treated with increasing concentrations of ZA, ranging from 10−4 to 10−5 M. After 3 days of exposure ZA induced decrease in proliferation and increase in apoptosis. Among the adhesion molecules, CD106, CD54, CD49d, and CD40, which were strongly expressed at baseline, showed a statistically significant reduction compared with controls after exposure to higher concentrations of ZA [37].
Bisphosphonate treatment in particular ZA and ibandronate inhibit the binding of human breast and prostate cancer cells to mineralized and unmineralized matrices [38], [39], [40].
Among possible anti-tumour mechanisms of ZA, the above mechanism seems to be the one least studied in pre-clinical setting. If this mechanism is confirmed in clinical setting, it may demonstrate an impact on development of bone metastases rather than direct anti-tumour effect.
1.1.5. Decrease in tumour cell invasion and migration, and disorganization of cell cytoskeleton
Tumour cell invasion is intrinsically linked to localized cell surface proteolytic activity driven by matrix metalloproteinases (MMPs), which facilitates cell detachment from matrix proteins, thereby promoting cell migration. Nitrogen-containing bisphosphonates (NBPs) inhibit breast and prostate cancer cell invasion [41], [42], [43], [44], [45]. High concentrations of bisphosphonates inhibit the zinc-dependent proteolytic activity of MMPs, whereas submicromolar concentrations are sufficient to inhibit tumour cell invasion [41]. Indeed, alendronate and ZA inhibit ovarian and breast cancer cell migration, by attenuating the geranylgeranylation of RhoA [42], [44], [45], [37], [38], [39], a key player in cell adhesion dynamics that drive cell motility. ZA also inhibits the chemokine CXCL12-induced breast cancer cell migration by decreasing the cell surface expression of CXCR4, the receptor for CXCL-12 [42], [37]. Therefore, the anti-invasive properties of NBPs may be the result of the inhibition of distinct molecular pathways that mediate cancer cell invasion in a coordinated fashion. These compounds may eventually also inhibit MMP activity if high local bisphosphonate concentrations are achieved in the tumour microenvironment [46].
Denoyelle et al. studied the effect of ZA on the invasiveness and morphology of MDA-MB-231 breast cancer cells. Treatment by ZA for 18 h induced significant inhibition of cell invasion starting at concentration of 1 μM with higher concentrations (10–100 μM) inducing more inhibition. One micromolar concentration of ZA was chosen to study the effect on cell morphology. Untreated cells were flat and well spread. In contrast ZA induced dramatic morphological changes characterized by a cell rounding and a disorganization of actin cytoskeleton accompanied by a loss of stress fibres formation [42]. The investigators found that the observed inhibition of cell invasion by ZA was associated with decreased translocation of RhoA from cytoplasm to the cell membrane.
Later work by different group of investigators demonstrated that ZA inhibits HUVEC adhesion, survival, migration and actin stress fibre formation by interfering with protein prenylation and has identified ERK1/2, JNK, Rock (RhoA target mediating actin fibre formation), FAK and PKB as kinases affected by ZA in a prenylation-dependent manner [47].
It remains to be seen if these anti-invasion and anti-migration effects of ZA combined with other anti-tumour effects can be translated into managing early stage breast cancer to prevent relapse and metastases.
1.1.6. Activation of γδ T cells
T cells bearing the T-cell receptor (TCR)- γδ represent a minor subset of human peripheral T cells (1–10%). The physiologic function of γδ T cells remains elusive, though some evidence suggests that γδ T cells play a role in the “first line of defence” against a broad spectrum of invasive microorganisms such as mycobacteria. In addition, certain hematopoietic tumour cells (e.g., Burkitt lymphoma cell line Daudi or myeloma cell line RPMI 8226) are specifically recognized and lysed by these T cells in vitro [48], [49]. The recognition of ubiquitous nonpeptide antigens by γδ T cells suggests a surveillance function of these T cells for infected or transformed cells [50].
One of the early studies examining the stimulatory capacity of bisphosphonates to γδ T cells was carried out by Kunzmann et al. In their work alendronate, ibandronate, and pamidronate induced significant expansion of γδ T cells (Vγ9Vδ2 subset) in peripheral blood mononuclear cell cultures of healthy donors. Pamidronate-activated γδ T cells produced cytokines (i.e., interferon [IFN]-γ) and exhibited specific cytotoxicity against lymphoma (Daudi) and myeloma cell lines (RPMI 8226, U266). Pamidronate-treated BM cultures of 24 patients with MM showed significantly reduced plasma cell survival compared with untreated cultures [51].
In another study examining the stimulatory capacity of ZA to γδ T cells, nine patients with metastatic bone disease from breast and prostate cancer were injected with ZA. Peripheral blood mononuclear cells were collected before treatment, and 1 month and 3 months after the first administration. The objective of this study was to evaluate the in vivo effect of ZA on subsets of Vγ9Vδ2 cells. The final conclusion was that in vivo treatment with ZA induces Vγ9Vδ2 cells to mature toward an IFNγ-producing effector phenotype, which may induce more effective antitumor responses [52].
Significant numbers of reports followed and confirmed the stimulatory effect of ZA on γδ T cells. ZA induced proliferation of γδ T cells from normal and MM patients. The cells produced IFN-γ and exerted direct cell-to-cell antimyeloma activity. ZA plus IL-2 increased the absolute number of γδ T cells 298-768 fold after 14 days incubation [53].
Several studies showed that ZA not only induced marked increase in sensitivity of tumour cells to lysis by γδ T cells [54], [55] but also increased the number of γδ T cell when IL-2 was co-administered [56]. The activating effect of ZA on γδ T cells was also observed in vivo in disease-free breast cancer patients after a single-dose of ZA [57]. This observation may serve as a rationale for clinical evaluation of ZA in the adjuvant setting. However, recent evidence suggests that repeated treatment with ZA in vivo reduces Vγ2Vδ2 T cell numbers and their responsiveness to stimulation [58]. This may explain the inconsistent results seen in large clinical trials investigating the role of ZA in adjuvant setting (as discussed below).
1.1.7. Effects on tumour macrophage infiltration
Tumour cells interact with the surrounding microenvironment to grow and develop. Macrophages are a major component of this microenvironment, and are of particular interest as potential therapeutic targets due to their central role in tumour progression.
It is accepted that tumour associated macrophages have an important role in several stages of oncogenesis and tumour progression including tumour cell growth, angiogenesis, migration, invasion, and metastasis [59]. Higher level of macrophage infiltration is associated with poor prognosis in several tumour types [60]. There is evidence that tumour macrophages play a role in regulating angiogenesis and thus may promote tumour cells proliferation [61].
There is in vivo and in vitro evidence that tumour macrophages uptake ZA. Exposure of J774 macrophages to 5 μM ZOL for 24 h caused increased uptake of ZA. Higher doses and longer exposure induced apoptotic cell death [62].
There is pre-clinical evidence that ZA is more potent macrophage inhibitor than other bisphosphonates. J774A.1 cells were cultured in a standard culture medium for 2-days. Bisphosphonates (alendronate, pamidronate, etidronate, risedronate and ZA) were added in the medium at concentration of 10−6–10−4 M during 3 days. Etidronate did not cause significant apoptosis or necrosis, at any concentration. Alendronate and pamidronate caused apoptosis and death only at very high concentration [10−4 M]. On the contrary, apoptotic and necrotic cells were evidenced with risedronate or ZA at lower concentrations [63].
Enhanced expression of Matrix Metalloprotinase-9 (MMP-9) in macrophages is induced by interaction with tumour cells. This plays a major role in tumour cells invasion and metastasis. In vivo, treatment with amino-biphosphonates was shown to impair tumour growth, decrease MMP-9 expression and decrease the number of macrophages in tumour stroma [64].
ZA has been found to reverse the polarity of peritoneal and tumour-associated macrophages from M2 to M1. This is a very important finding, as M1 macrophages possess tumoricidal activity, supporting that TAMS are a potential immune target of ZOL therapy [65].
The macrophage mediated anti-tumour effect of amino-biphosphonates is well established in pre-clinical studies. However, there is lack of clinical studies confirming this effect in cancer patients.
1.2. Early clinical (phase I/II) data regarding anticancer effects of zoledronic acid
1.2.1. Direct cytotoxic effect of ZA
As discussed above, preclinical evidence suggests that bisphosphonates (with special reference to nitrogen containing bisphosphonates including ZA) possess inherent anti-tumour activity. These data suggest that adding ZA to other systemic cancer therapies may provide additional antitumor and anti-metastatic activity.
In the clinical setting, the results of large clinical trials investigating bisphosphonates less potent than ZA (clodronate and pamidronate) were inconsistent [66], [67]. Because of these potential antitumor effects, ZA which is more potent than clodronate and pamidronate has been investigated in early clinical studies in cancer patients with and without metastases.
In one study, 40 patients with recurrent or metastatic advanced cancer, without bone metastases, were randomized to receive ZA or no treatment. Patients were followed up until bone metastases were established. At 1 year, 60% of patients in ZA arm compared to 10% in the control group were bone metastases free (p<0.0005). At 18 months, the percentages were 20% and 5% (p=0.0002) [68].
In a phase II trial, 120 women with clinical stage II–III breast cancer planned for four cycles of neoadjuvant chemotherapy were randomised to receive 4 mg ZA intravenously every 3 weeks or no ZA for 1 year. The primary endpoint was the number of patients with detectable disseminated tumour cells (DTCs) at 3 months. At baseline, DTCs were detected in 43% of patients in the ZA group and 48% of patients in the control group. At 3 months, 30% patients receiving ZA versus 47% of patients who did not receive ZA had detectable DTCs (p=0.054) [69]. The antitumour effect of ZA on bone marrow DTC in women with early breast cancer was also shown by other investigators [70], [71].
1.2.2. Inhibition of angiogenesis
In an Italian study, 42 breast cancer patients with bone metastases were treated with a single infusion of 4 mg ZA before anticancer chemotherapy. The patients were prospectively evaluated for circulating levels of VEGF just before and at 1, 2, 7, and 21 days after ZA infusion. Serum VEGF median levels were significantly decreased at each time point after treatment, but the major reduction was recorded 21 days after the infusion. Twenty-five patients (59.5%) experienced a reduction of at least 25% in the VEGF circulating levels. The analysis of survival showed that patients with a reduction in VEGF circulating levels had longer time to first skeletal-related event (p=0.0002), time to bone progression disease (p=0.0024), and time to performance status worse (p=0.0352) than those without the VEGF reduction. No statistically significant differences were recorded in terms of overall survival and time to visceral progression [72]. This study confirms that ZA has an in vivo antiangiogenic property and that VEGF modifications may represent a surrogate marker to outcome.
These results were repeated by the same group in another 24 patients. Moreover, there was significant correlation between median VEGF and the bone resorption marker betaCTX [73].
Interestingly, in both studies [72], [73] serum VEGF levels showed sustained suppression after a single dose of ZA. This is in parallel to the finding that repeated treatment with ZA in vivo reduces Vγ2Vδ2 T cell numbers and their responsiveness to stimulation [58]. These findings raise the question of the number of ZA doses that should be considered optimal for future clinical trials.
Reduction in circulating VEGF appeared to be long lasting and persisted as long as patients were receiving ZA [74]. In a study of 60 breast cancer patients with bone metastases, metronomic ZA was more effective than the conventional regimen and generated sustained reductions in circulating VEGF and bone resorption marker levels, as well as stabilization of serum CA 15-3 levels [75], [61]. It is interesting that metronomic dose of ZA (1 mg weekly) also reduced serum level of VEGF in cancer patients in other studies [74], [75]. Other early clinical studies showed different results. In a study with 18 breast cancer patients with bone metastases, serum VEGF level showed a statistically significant decrease 48 h after ZA infusion but rose above the basal level at 7 days [76]. In another study of 30 patients with metastatic bone disease from breast and lung cancer ZA acid did not exert significant reduction of VEGF and bFGF circulating levels 7 days after treatment [77]. These studies further bring to question the optimal dose and frequency of ZA that should be used in future clinical trials.
1.2.3. Activation of γδ T cells
A phase I study showed that administration of ZA (with IL-2) to patients with metastatic cancer promoted effector maturation of γδ T cells. Three patients who showed sustained robust peripheral γδ T cell populations had decrease in their tumour marker levels, with one patient achieving partial response and two achieving stable disease. In contrast, seven other patients who failed to sustain peripheral γδ T cells showed clinical deterioration [78].
In a Japanese study, peripheral blood mononuclear cells from 15 patients with advanced non-small cell lung cancer (NSCLC) were stimulated with ZA (5 μM) and IL-2 (1000 IU/ml) for 14 days. Many of these cells expanded to γδ T cells. Administration of these ex vivo expanded γδ T cells to patients with recurrent or advanced lung cancer was well tolerated. The number of peripheral γδ T cells gradually increased. All patients remained alive during the study period with a median overall survival of 589 (range 202–1505) days, and median progression free survival of 126 (range 34–285) days [79]. According to the Response Evaluation Criteria in Solid Tumours (RECIST), there were no objective responses. Six patients had stable disease, whereas the remaining six evaluable patients experienced progressive disease 4 weeks after the sixth transfer.
Although all of these patients had Eastern Cooperative Group (ECOG) performance status 0 or 1 with minimal co-morbidities, such a long period of survival is very encouraging for recurrent or advanced lung cancer. The reported median survival in this study is about 1.5 times longer than that reported in large phase III lung cancer trial.
Results from other studies were less promising. For example, in a study of 12 patients with renal cell carcinoma, ZA induced only modest increase in γδ T cell frequency but not to the magnitude anticipated from preclinical models [80].
Recently, investigators have shed some light on possible reason for the lack objective responses of solid tumours to γδ T cells expansion therapy. Using similar expansion technique, Kunzmann et al. treated 21 adults with advanced malignancies (renal cell carcinoma [RCC], malignant melanoma, and acute myeloid leukaemia). No objective responses were observed in both cohorts of solid tumours (RCC and malignant melanoma), whereas two patients with acute myeloid leukaemia (25%) achieved objective tumour responses. Pharmacodynamic analyses showed significant in vivo activation (interferon-γ production) and expansion of γδ T cells in all evaluable patients. High pretreatment serum VEGF levels and an unexpected increase in VEGF induced ZA plus low-dose interleukin-2 were correlated with the lack of a clinical response. This study indicates that immunotherapy-induced VEGF can limit clinical innate tumour immune responses, especially for angiogenesis-dependent solid tumours. Adoptive immunotherapy in this context is still at its infancy and further early clinical studies may elucidate its potential role.
1.3. Phase III trials of zoledronic acid in early breast cancer
Based on early clinical data suggesting its anti-tumour effect, ZA has been studied in multiple large randomized clinical trials in early breast cancer.
In an Austrian study (ABCSG-12) [81], 1803 endocrine responsive early breast cancer patients on adjuvant goserelin were randomly assigned to receive, tamoxifen or anastrozole, with or without ZA. The dose of ZA was 4 mg given intravenously every 6 months for 3 years. There was a 36% decrease in the risk of disease progression (p=0.01) in the ZA arms. This corresponded to a 5 year disease free survival of 94% versus 90.8%, favouring ZA. There were also fewer deaths in the ZA arms (16 versus 26, hazard ratio 0.60, p=0.11). Interestingly, patients on the ZA arms had fewer events in all examined categories including loco regional recurrence (10 versus 20), distant recurrence (29 versus 41), and contra lateral breast cancer (6 versus 10) as compared to treatment without ZA.
An integrated analysis [82] of two large randomized controlled trials (Z-FAST and Zo-FAST) treating a total of 1667 patients with early breast cancer was performed. The patients on these studies were randomized to receive adjuvant hormonal therapy (letrozole), with either upfront ZA or delayed ZA (at time of decreased bone mineral density). The analysis was aimed primarily to assess the protective effect of ZA in prevention of bone density loss. One of the secondary objectives of the analysis was time to disease recurrence. The results showed that patients treated with upfront ZA had significantly less recurrences at 12 months as compared to the patients who received delayed ZA (0.84% versus 1.9%, p=0.04). An updated analysis of the ZO-FAST study at 36 months showed continued benefit of upfront ZA in prolonging disease free survival (41% relative risk reduction, p=0.04) as compared to delayed ZA [83]. A more recent analysis at 60 months showed continued benefit of prolonged disease free survival (HR0.66, p=0.0375) in the upfront ZA group [84].
A third study (AZURE) [24] randomized 3360 patients with early breast cancer to receive standard adjuvant therapy with or without ZA. ZA was administered immediately after each cycle of adjuvant chemotherapy in line with pre-clinical data supporting this sequence as discussed above and was continued for a total of 5 years.
At a median follow up of 59 months, the investigators found no significant difference between the two arms in the primary end-point which was disease free survival (77% in each group, p=0.79). Prospective subgroup analyses of the study showed that ZA significantly increased disease free survival (HR=0.76, p<0.05) in women who were at least 5 years past menopause at study entry (n=1041). ZA also improved overall survival (HR=0.71, p=0.017, n=1101) in this patient population along with women of unknown postmenopausal status but age greater than 60 years [85]. Of note, about 45.8% of patients in this study were premenopausal, 95% of whom received chemotherapy, and only 0.2% of these patients received goserelin. This is in contrast to the pre-menopausal patient cohort in the ABCSG-12 trial, who all received goserelin.
Apparently the results of the AZURE study are in contradiction to the ABCSG-12 results. However, some authorities have argued that in both studies, disease free and overall survival was seen in patients who were either rendered menopausal (with goserelin in ABCSG-12 trial), or who achieved menopause naturally (as seen in the subset analyses of the AZURE trial), suggesting a possible relationship of a menopausal or low oestrogen state with the clinical benefit achieved from addition of ZA [86].
205 Patients in the AZURE study received neo-adjuvant treatment including ZA. A retrospective analysis of pathologic response in these patients [23] showed that the mean residual tumour size in the ZA plus chemotherapy arm was significantly less (15.5 mm) as compared to 27.4 mm in the chemotherapy only arm. The pathologic complete response rate was 11.7% in the ZA arm versus 6.9% in the chemotherapy only arm, but this was not statistically significant (p=0.146).
Although the ABCSG-12 and AZURE studies have provided provocative and interesting conclusions, the partly contradicting results of these trials has delayed the incorporation of ZA in the adjuvant treatment of women with early breast cancer.
A prospective study in postmenopausal patients is required to validate and confirm the results of the ABCSG-12 and AZURE trials. This will also be an opportunity to study specific biomarkers that likely explain anti-tumour mechanism of ZA. As discussed above, anti-angiogenesis and anti-proliferative properties of ZA seem to be the most relevant mechanisms from early preclinical and small clinical studies.
Several other large randomized controlled trials are evaluating the anti-tumour efficacy of ZA in the adjuvant setting (Table 2). Most of these studies have completed accrual and are awaiting maturation of the data.
Table 2.
A summary of ongoing large clinical trials evaluating the use of Zoledronic acid in different cancers.
| Study name | Patient population | Study design and treatment | Primary endpoints | References |
|---|---|---|---|---|
| NATAN | 654 BC patients | Standard therapy +/− | EFS at 5 y | ClinicaTrials.gov No. NCT00512993 |
| ZA (4 mg IV q1mo; q3mo; q6mo) | German Breast Group | |||
| SUCCESS | 3754 BC patients (stages I, II, and IIIA) | FEC/DOC +/− GEM, then endocrine therapy plus ZA for 2 y or 5 y | DFS at 5 y | The SUCCESS Study Group 2009 |
| www.Success-studie.de | ||||
| SWOG 0307 | 5400 BC patients (stages I, II, and IIIA) | ZA (4 mg q1mo; q3mo); CLO (1600 mg/d); IBN (50 mg/d) | DFS and OS up to 10 y | ClinicTrials.gov No. NCT00127205 |
| Southwest Oncology Group | ||||
| ZEUS | 1498 PC patients (high-risk, early) | Standard therapy +/− ZA (4 mg IV q3mo) | EFS at 4 y | Register no. 66626762 〈http://www.controlled-trials.com/〉 |
| European Association of Urology | ||||
| RADAR | 1071 PC (stage T2b-4) | ADT for 6 mo or 18 mo +/− ZA (4 mg IV q3mo) | PSA-RFS at 18 mo | ClinicalTrials.gov No. NCT00193856 |
| Trans-Tasman Radiation Oncology Group | ||||
| STAMPEDE | 3300 PC patients (high risk) | ADT and (1) no additional therapy; (2) DOC (q3wk); (3) celecoxib (BID); (4) ZA (q3wk; q4wk); (5) DOC+ZOL; (6) celecoxib+ZA | FFS and OS (multiple phases) | ClinicalTrials.gov Identifier, NCT00268476 |
| Medical Research Council | ||||
| Study 2419 | 446 NSCLC patients (stage IIIA or IIIB) | ZA (4 mg IV q1mo) | TTP | ClinicalTrials.gov Identifier NCT00172042 |
| Novartis 2009 | ||||
| CALGB 90202 | 680 pts with met PrCa on ADT | ZA 4 mg IV every month versus placebo | PFS and OS | ClinicalTrials.gov Identifier: NCT00079001 |
BC indicates breast cancer; IBN, ibandronate; IV, intravenous; q4wk, every 4 weeks; +/−, with or without; DFS, disease-free survival; FEC, combined 5-flurouracil, epirubicin, and cyclophosphamide; DOC, docetaxel; ZA, zoledronic acid; EFS, event-free survival; q1mo, monthly; q3mo, every 3 months; GEM, gemcitabine; OS, overall survival; CLO, clodronate; PC, prostate cancer; ADT, androgen-deprivation therapy; PSA-RFS, prostate-specific antigen recurrence-free survival; BID, twice daily; FFS, failure free survival; NSCLC, non-small cell lung cancer; TTP, time to progression.
1.4. Trials showing lack of benefit from zoledronic acid
Although the above mentioned trials have shown some clinical benefit from the use of ZA in solid tumours, results from other trials do not show clear benefit from use of ZA. In a recent Italian study [87], patients with controlled stage IIIA/B NSCLC were randomized to receive ZA or no treatment. Progression free survival (PFS), which was the primary endpoint of this trial, was 9 months for ZA versus 11.3 months for control (statistically non-significant difference). Rate of development of bone metastases was also similar in both arms (6.6% in ZA arm and 9% in control arm, no significant difference).
In an open label randomized phase II study, 119 female patients with resectable stage II/III breast cancer [88], patients were randomised to either receive ZA or no ZA starting with first cycle of chemotherapy. At 61.9 months of follow up, there was no difference between the two arms in terms of disease free survival (secondary end-point) or overall survival (tertiary end-point). A subset analysis did show benefit of ZA in improving disease free survival (Hazard ratio 0.361, 95% confidence interval 0.148–0.880) and overall survival (Hazard ratio 0.375, 95% confidence interval 0.143–0.985) in patients whose tumours were oestrogen receptor negative as opposed to oestrogen receptor positive, however the study was not powered for this comparison. The authors suggest that in oestrogen receptor negative breast cancer, ZA may have a direct anti-tumour effect which may not be dependent on a low oestrogen environment. The sample size in these two clinical studies was too small to address the question of effect of ZA on disease relapse in early cancer setting.
1.5. Other bone modifying agents in oncology
In addition to ZA and other bisphosphonates, there is a host of other bone modifying agents in different phases of development. These include (but are not limited to) Denosumab (a RANK-ligand antibody), 223RaCl2 or Alpharadin (an alpha particle emitting agent), and antibodies to sclerostin (which is an inhibitor of osteoblastogenesis) [89]. The detailed mechanisms of action of these agents and whether they have anti-tumour effects are beyond the scope of this review.
2. Conclusion
ZA has become an important component in treatment of cancer patients. Apart from its role in bone preservation and reduction of skeletal related events, there is preclinical and also clinical evidence suggesting a direct anti-cancer effect of ZA. Several mechanisms of action for this anti-tumour effect have been studied. The most profound clinical evidence of this effect comes from a number of large adjuvant breast cancer studies, although the results have not been consistently in favour of antitumour effect of ZA. Other adjuvant studies incorporating ZA are currently underway and those results are awaited.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Contributor Information
Jamal Zekri, Email: jzekri@kfshrc.edu.sa.
Maged Mansour, Email: magedmansour@hotmail.com.
Syed Mustafa Karim, Email: skarim@kfshrc.edu.sa.
References
- 1.Hosfield D.J., Zhang Y., Dougan D.R., Broun A., Tari L.W., Swanson R.V. Structural basis for bisphosphonate-mediated inhibition of isoprenoid biosynthesis. J Biol Chem. 2004;279(10):8526–8529. doi: 10.1074/jbc.C300511200. [DOI] [PubMed] [Google Scholar]
- 2.Rogers M.J. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif Tissue Int. 2004;75(6):451–461. doi: 10.1007/s00223-004-0024-1. [DOI] [PubMed] [Google Scholar]
- 3.Reinholz G.G., Getz B., Sanders E.S., Karpeisky M.Y., Padyukova N., Mikhailov S.N. Distinct mechanisms of bisphosphonate action between osteoblasts and breast cancer cells: identity of a potent new bisphosphonate analogue. Breast Cancer Res Treat. 2002;71(3):257–268. doi: 10.1023/a:1014418017382. [DOI] [PubMed] [Google Scholar]
- 4.Dunford J.E., Thompson K., Coxon F.P., Luckman S.P., Hahn F.M., Poulter C.D. Structure-activity relationships for inhibition of farnesyl diphosphate synthase in vitro and inhibition of bone resorption in vivo by nitrogen-containing bisphosphonates. J Pharmacol Exp Ther. 2001;296(2):235–242. [PubMed] [Google Scholar]
- 5.Luckman S.P., Hughes D.E., Coxon F.P., Graham R., Russell G., Rogers M.J. Nitrogen-containing bisphosphonates inhibit the mevalonate pathway and prevent post-translational prenylation of GTP-binding proteins, including Ras. J Bone Miner Res. 1998;13(4):581–589. doi: 10.1359/jbmr.1998.13.4.581. [DOI] [PubMed] [Google Scholar]
- 6.Koto K., Murata H., Kimura S., Horie N., Matsui T., Nishigaki Y. Zoledronic acid inhibits proliferation of human fibrosarcoma cells with induction of apoptosis, and shows combined effects with other anticancer agents. Oncol Rep. 2010;24(1):233–239. doi: 10.3892/or_00000851. [DOI] [PubMed] [Google Scholar]
- 7.Koto K., Horie N., Kimura S., Murata H., Sakabe T., Matsui T. Clinically relevant dose of zoledronic acid inhibits spontaneous lung metastasis in a murine osteosarcoma model. Cancer Lett. 2009;274(2):271–278. doi: 10.1016/j.canlet.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 8.Zwolak P., Manivel J.C., Jasinski P., Kirstein M.N., Dudek A.Z., Fisher J. Cytotoxic effect of zoledronic acid-loaded bone cement on giant cell tumor, multiple myeloma, and renal cell carcinoma cell lines. J Bone Joint Surg Am. 2010;92(1):162–168. doi: 10.2106/JBJS.H.01679. [DOI] [PubMed] [Google Scholar]
- 9.Tamura T., Shomori K., Nakabayashi M., Fujii N., Ryoke K., Ito H. Zoledronic acid, a third-generation bisphosphonate, inhibits cellular growth and induces apoptosis in oral carcinoma cell lines. Oncol Rep. 2011;25(4):1139–1143. doi: 10.3892/or.2011.1152. [DOI] [PubMed] [Google Scholar]
- 10.Ullen A., Schwarz S., Lennartsson L., Kalkner K.M., Sandstrom P., Costa F. Zoledronic acid induces caspase-dependent apoptosis in renal cancer cell lines. Scand J Urol Nephrol. 2009;43(2):98–103. doi: 10.1080/00365590802475904. [DOI] [PubMed] [Google Scholar]
- 11.Koizumi M., Nakaseko C., Ohwada C., Takeuchi M., Ozawa S., Shimizu N. Zoledronate has an antitumor effect and induces actin rearrangement in dexamethasone-resistant myeloma cells. Eur J Haematol. 2007;79(5):382–391. doi: 10.1111/j.1600-0609.2007.00957.x. [DOI] [PubMed] [Google Scholar]
- 12.Di Salvatore M., Orlandi A., Bagala C., Quirino M., Cassano A., Astone A. Anti-tumour and anti-angiogenetic effects of zoledronic acid on human non-small-cell lung cancer cell line. Cell Prolif. 2011;44(2):139–146. doi: 10.1111/j.1365-2184.2011.00745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Almubarak H., Jones A., Chaisuparat R., Zhang M., Meiller T.F., Scheper M.A. Zoledronic acid directly suppresses cell proliferation and induces apoptosis in highly tumorigenic prostate and breast cancers. J Carcinog. 2011;10:2. doi: 10.4103/1477-3163.75723. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Ullen A., Lennartsson L., Harmenberg U., Hjelm-Eriksson M., Kalkner K.M., Lennernas B. Additive/synergistic antitumoral effects on prostate cancer cells in vitro following treatment with a combination of docetaxel and zoledronic acid. Acta Oncol. 2005;44(6):644–650. doi: 10.1080/02841860510029617. [DOI] [PubMed] [Google Scholar]
- 15.Karabulut B., Erten C., Gul M.K., Cengiz E., Karaca B., Kucukzeybek Y. Docetaxel/zoledronic acid combination triggers apoptosis synergistically through downregulating antiapoptotic Bcl-2 protein level in hormone-refractory prostate cancer cells. Cell Biol Int. 2009;33(2):239–246. doi: 10.1016/j.cellbi.2008.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Jagdev S.P., Coleman R.E., Shipman C.M., Rostami H.A., Croucher P.I. The bisphosphonate, zoledronic acid, induces apoptosis of breast cancer cells: evidence for synergy with paclitaxel. Br J Cancer. 2001;84(8):1126–1134. doi: 10.1054/bjoc.2001.1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ozturk O.H., Bozcuk H., Burgucu D., Ekinci D., Ozdogan M., Akca S. Cisplatin cytotoxicity is enhanced with zoledronic acid in A549 lung cancer cell line: preliminary results of an in vitro study. Cell Biol Int. 2007;31(9):1069–1071. doi: 10.1016/j.cellbi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 18.Ottewell P.D., Monkkonen H., Jones M., Lefley D.V., Coleman R.E., Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100(16):1167–1178. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 19.Neville-Webbe H.L., Coleman R.E., Holen I. Combined effects of the bisphosphonate, zoledronic acid and the aromatase inhibitor letrozole on breast cancer cells in vitro: evidence of synergistic interaction. Br J Cancer. 2010;102(6):1010–1017. doi: 10.1038/sj.bjc.6605579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Neville‐Webbe H.L., Rostami‐Hodjegan A., Evans C.A., Coleman R.E., Holen I. Sequence‐and schedule‐dependent enhancement of zoledronic acid induced apoptosis by doxorubicin in breast and prostate cancer cells. Int J Cancer. 2005;113(3):364–371. doi: 10.1002/ijc.20602. [DOI] [PubMed] [Google Scholar]
- 21.Charehbili Ayoub, van deVen Saskia, Gerrit-Jan Liefers. NEOZOTAC: Efficacy results from a phase III randomized trial with neoadjuvant chemotherapy (TAC) with or without zoledronic acid for patients with HER2-negative large resectable or stage II or III breast cancer (BC)—a Dutch Breast Cancer Trialists′ Group (BOOG) study. J Clin Oncol. 2013;31(suppl) Abstract 1028. [Google Scholar]
- 22.Winter M.C., Wilson C., Syddall S.P., Cross S.S., Evans A., Ingram C.E. Neoadjuvant chemotherapy with or without zoledronic acid in early breast cancer—a randomized biomarker pilot study. Clin Cancer Res. 2013;19(10):2755–2765. doi: 10.1158/1078-0432.CCR-12-3235. [DOI] [PubMed] [Google Scholar]
- 23.Coleman R.E., Winter M.C., Cameron D., Bell R., Dodwell D., Keane M.M. The effects of adding zoledronic acid to neoadjuvant chemotherapy on tumour response: exploratory evidence for direct anti-tumour activity in breast cancer. Br J Cancer. 2010;102(7):1099–1105. doi: 10.1038/sj.bjc.6605604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coleman R.E., Marshall H., Cameron D., Dodwell D., Burkinshaw R., Keane M. Breast-cancer adjuvant therapy with zoledronic acid. N Engl J Med. 2011;365(15):1396–1405. doi: 10.1056/NEJMoa1105195. [DOI] [PubMed] [Google Scholar]
- 25.Folkman J. Tumor angiogenesis: therapeutic implications. N Engl J Med. 1971;285(21):1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 26.Yamada J., Tsuno N.H., Kitayama J., Tsuchiya T., Yoneyama S., Asakage M. Anti-angiogenic property of zoledronic acid by inhibition of endothelial progenitor cell differentiation. J Surg Res. 2009;151(1):115–120. doi: 10.1016/j.jss.2008.01.031. [DOI] [PubMed] [Google Scholar]
- 27.Wood J., Bonjean K., Ruetz S., Bellahcene A., Devy L., Foidart J.M. Novel antiangiogenic effects of the bisphosphonate compound zoledronic acid. J Pharmacol Exp Ther. 2002;302(3):1055–1061. doi: 10.1124/jpet.102.035295. [DOI] [PubMed] [Google Scholar]
- 28.Ziebart T., Pabst A., Klein M.O., Kammerer P., Gauss L., Brullmann D. Bisphosphonates: restrictions for vasculogenesis and angiogenesis: inhibition of cell function of endothelial progenitor cells and mature endothelial cells in vitro. Clin Oral Investig. 2011;15(1):105–111. doi: 10.1007/s00784-009-0365-2. [DOI] [PubMed] [Google Scholar]
- 29.Misso G., Porru M., Stoppacciaro A., Castellano M., De Cicco F., Leonetti C. Evaluation of the in vitro and in vivo antiangiogenic effects of denosumab and zoledronic acid. Cancer Biol Ther. 2012;13(14):1491–1500. doi: 10.4161/cbt.22274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsai S.-H., Huang P.-H., Chang W.-C., Tsai H.-Y., Lin C.-P., Leu H.-B. Zoledronate inhibits ischemia-induced neovascularization by impairing the mobilization and function of endothelial progenitor cells. PloS One. 2012;7(7):e41065. doi: 10.1371/journal.pone.0041065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stresing V., Fournier P.G., Bellahcene A., Benzaid I., Monkkonen H., Colombel M. Nitrogen-containing bisphosphonates can inhibit angiogenesis in vivo without the involvement of farnesyl pyrophosphate synthase. Bone. 2011;48(2):259–266. doi: 10.1016/j.bone.2010.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Kuroshima S., Elliott K.W., Yamashita J. Effect of zoledronate on the responses of osteocytes to acute parathyroid hormone. Calcif Tissue Int. 2013;92(6):576–585. doi: 10.1007/s00223-013-9720-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kobayashi Y., Hiraga T., Ueda A., Wang L., Matsumoto-Nakano M., Hata K. Zoledronic acid delays wound healing of the tooth extraction socket, inhibits oral epithelial cell migration, and promotes proliferation and adhesion to hydroxyapatite of oral bacteria, without causing osteonecrosis of the jaw, in mice. J Bone Miner Metab. 2010;28(2):165–175. doi: 10.1007/s00774-009-0128-9. [DOI] [PubMed] [Google Scholar]
- 34.Aragon-Ching J.B., Ning Y.M., Chen C.C., Latham L., Guadagnini J.P., Gulley J.L. Higher incidence of Osteonecrosis of the Jaw (ONJ) in patients with metastatic castration resistant prostate cancer treated with anti-angiogenic agents. Cancer Invest. 2009;27(2):221–226. doi: 10.1080/07357900802208608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Christodoulou C., Pervena A., Klouvas G., Galani E., Falagas M.E., Tsakalos G. Combination of bisphosphonates and antiangiogenic factors induces osteonecrosis of the jaw more frequently than bisphosphonates alone. Oncology. 2009;76(3):209–211. doi: 10.1159/000201931. [DOI] [PubMed] [Google Scholar]
- 36.David Roodman G. Role of stromal-derived cytokines and growth factors in bone metastasis. Cancer. 2003;97(Suppl. 3):S733–S738. doi: 10.1002/cncr.11148. [DOI] [PubMed] [Google Scholar]
- 37.Corso A., Ferretti E., Lunghi M., Zappasodi P., Mangiacavalli S., De Amici M. Zoledronic acid down-regulates adhesion molecules of bone marrow stromal cells in multiple myeloma: a possible mechanism for its antitumor effect. Cancer. 2005;104(1):118–125. doi: 10.1002/cncr.21104. [DOI] [PubMed] [Google Scholar]
- 38.Boissier S., Magnetto S., Frappart L., Cuzin B., Ebetino F.H., Delmas P.D. Bisphosphonates inhibit prostate and breast carcinoma cell adhesion to unmineralized and mineralized bone extracellular matrices. Cancer Res. 1997;57(18):3890–3894. [PubMed] [Google Scholar]
- 39.Magnetto S., Boissier S., Delmas P.D., Clezardin P. Additive antitumor activities of taxoids in combination with the bisphosphonate ibandronate against invasion and adhesion of human breast carcinoma cells to bone. Int J Cancer. 1999;83(2):263–269. doi: 10.1002/(sici)1097-0215(19991008)83:2<263::aid-ijc19>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 40.van der Pluijm G., Vloedgraven H., van Beek E., van der Wee-Pals L., Lowik C., Papapoulos S. Bisphosphonates inhibit the adhesion of breast cancer cells to bone matrices in vitro. J Clin Invest. 1996;98(3):698–705. doi: 10.1172/JCI118841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Clezardin P., Fournier P., Boissier S., Peyruchaud O. In vitro and in vivo antitumor effects of bisphosphonates. Curr Med Chem. 2003;10(2):173–180. doi: 10.2174/0929867033368529. [DOI] [PubMed] [Google Scholar]
- 42.Denoyelle C., Hong L., Vannier J.P., Soria J., Soria C. New insights into the actions of bisphosphonate zoledronic acid in breast cancer cells by dual RhoA-dependent and -independent effects. Br J Cancer. 2003;88(10):1631–1640. doi: 10.1038/sj.bjc.6600925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Green J.R. Antitumor effects of bisphosphonates. Cancer. 2003;97(Suppl. 3):S840–S847. doi: 10.1002/cncr.11128. [DOI] [PubMed] [Google Scholar]
- 44.Hiraga T., Williams P.J., Ueda A., Tamura D., Yoneda T. Zoledronic acid inhibits visceral metastases in the 4T1/luc mouse breast cancer model. Clin Cancer Res. 2004;10(13):4559–4567. doi: 10.1158/1078-0432.CCR-03-0325. [DOI] [PubMed] [Google Scholar]
- 45.Sawada K., Morishige K., Tahara M., Kawagishi R., Ikebuchi Y., Tasaka K. Alendronate inhibits lysophosphatidic acid-induced migration of human ovarian cancer cells by attenuating the activation of rho. Cancer Res. 2002;62(21):6015–6020. [PubMed] [Google Scholar]
- 46.Clezardin P., Ebetino F.H., Fournier P.G. Bisphosphonates and cancer-induced bone disease: beyond their antiresorptive activity. Cancer Res. 2005;65(12):4971–4974. doi: 10.1158/0008-5472.CAN-05-0264. [DOI] [PubMed] [Google Scholar]
- 47.Hasmim M., Bieler G., Ruegg C. Zoledronate inhibits endothelial cell adhesion, migration and survival through the suppression of multiple, prenylation-dependent signaling pathways. J Thromb Haemost. 2007;5(1):166–173. doi: 10.1111/j.1538-7836.2006.02259.x. [DOI] [PubMed] [Google Scholar]
- 48.Bukowski J.F., Morita C.T., Tanaka Y., Bloom B.R., Brenner M.B., Band H. V gamma 2V delta 2 TCR-dependent recognition of non-peptide antigens and Daudi cells analyzed by TCR gene transfer. J Immunol. 1995;154(3):998–1006. [PubMed] [Google Scholar]
- 49.Fisch P., Malkovsky M., Kovats S., Sturm E., Braakman E., Klein B.S. Recognition by human V gamma 9/V delta 2 T cells of a GroEL homolog on Daudi Burkitt′s lymphoma cells. Science. 1990;250(4985):1269–1273. doi: 10.1126/science.1978758. [DOI] [PubMed] [Google Scholar]
- 50.De Libero G. Sentinel function of broadly reactive human gamma delta T cells. Immunol Today. 1997;18(1):22–26. doi: 10.1016/s0167-5699(97)80010-2. [DOI] [PubMed] [Google Scholar]
- 51.Kunzmann V., Bauer E., Feurle J., Weissinger F., Tony H.P., Wilhelm M. Stimulation of gammadelta T cells by aminobisphosphonates and induction of antiplasma cell activity in multiple myeloma. Blood. 2000;96(2):384–392. [PubMed] [Google Scholar]
- 52.Dieli F., Gebbia N., Poccia F., Caccamo N., Montesano C., Fulfaro F. Induction of gammadelta T-lymphocyte effector functions by bisphosphonate zoledronic acid in cancer patients in vivo. Blood. 2003;102(6):2310–2311. doi: 10.1182/blood-2003-05-1655. [DOI] [PubMed] [Google Scholar]
- 53.Mariani S., Muraro M., Pantaleoni F., Fiore F., Nuschak B., Peola S. Effector gammadelta T cells and tumor cells as immune targets of zoledronic acid in multiple myeloma. Leukemia. 2005;19(4):664–670. doi: 10.1038/sj.leu.2403693. [DOI] [PubMed] [Google Scholar]
- 54.Marten A., Lilienfeld-Toal M., Buchler M.W., Schmidt J. Zoledronic acid has direct antiproliferative and antimetastatic effect on pancreatic carcinoma cells and acts as an antigen for delta2 gamma/delta T cells. J Immunother. 2007;30(4):370–377. doi: 10.1097/CJI.0b013e31802bff16. [DOI] [PubMed] [Google Scholar]
- 55.Sato K., Kimura S., Segawa H., Yokota A., Matsumoto S., Kuroda J. Cytotoxic effects of gammadelta T cells expanded ex vivo by a third generation bisphosphonate for cancer immunotherapy. Int J Cancer. 2005;116(1):94–99. doi: 10.1002/ijc.20987. [DOI] [PubMed] [Google Scholar]
- 56.Naoe M., Ogawa Y., Takeshita K., Morita J., Shichijo T., Fuji K. Zoledronate stimulates gamma delta T cells in prostate cancer patients. Oncol Res. 2010;18(10):493–501. doi: 10.3727/096504010x12671222663638. [DOI] [PubMed] [Google Scholar]
- 57.Santini D., Martini F., Fratto M.E., Galluzzo S., Vincenzi B., Agrati C. In vivo effects of zoledronic acid on peripheral gammadelta T lymphocytes in early breast cancer patients. Cancer Immunol Immunother. 2009;58(1):31–38. doi: 10.1007/s00262-008-0521-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sugie T., Murata-Hirai K., Iwasaki M., Morita C.T., Li W., Okamura H. Zoledronic acid-induced expansion of gammadelta T cells from early-stage breast cancer patients: effect of IL-18 on helper NK cells. Cancer Immunol Immunother. 2013;62(4):677–687. doi: 10.1007/s00262-012-1368-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Allavena P., Sica A., Solinas G., Porta C., Mantovani A. The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66(1):1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 60.Coffelt S.B., Hughes R., Lewis C.E. Tumor-associated macrophages: effectors of angiogenesis and tumor progression. Biochim Biophys Acta. 2009;1796(1):11–18. doi: 10.1016/j.bbcan.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 61.Lin E.Y., Pollard J.W. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67(11):5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 62.Rogers T.L., Wind N., Hughes R., Nutter F., Brown H.K., Vasiliadou I. Macrophages as potential targets for zoledronic acid outside the skeleton-evidence from in vitro and in vivo models. Cell Oncol (Dordr) 2013;36(6):505–514. doi: 10.1007/s13402-013-0156-2. [DOI] [PubMed] [Google Scholar]
- 63.Moreau M.F., Guillet C., Massin P., Chevalier S., Gascan H., Basle M.F. Comparative effects of five bisphosphonates on apoptosis of macrophage cells in vitro. Biochem Pharmacol. 2007;73(5):718–723. doi: 10.1016/j.bcp.2006.09.031. [DOI] [PubMed] [Google Scholar]
- 64.Melani C., Sangaletti S., Barazzetta F.M., Werb Z., Colombo M.P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 2007;67(23):11438–11446. doi: 10.1158/0008-5472.CAN-07-1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Coscia M., Quaglino E., Iezzi M., Curcio C., Pantaleoni F., Riganti C. Zoledronic acid repolarizes tumour-associated macrophages and inhibits mammary carcinogenesis by targeting the mevalonate pathway. J Cell Mol Med. 2010;14(12):2803–2815. doi: 10.1111/j.1582-4934.2009.00926.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ha T.C., Li H. Meta-analysis of clodronate and breast cancer survival. Br J Cancer. 2007;96(12):1796–1801. doi: 10.1038/sj.bjc.6603661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kristensen B., Ejlertsen B., Mouridsen H.T., Jensen M.B., Andersen J., Bjerregaard B. Bisphosphonate treatment in primary breast cancer: results from a randomised comparison of oral pamidronate versus no pamidronate in patients with primary breast cancer. Acta Oncol. 2008;47(4):740–746. doi: 10.1080/02841860801964988. [DOI] [PubMed] [Google Scholar]
- 68.Mystakidou K., Katsouda E., Parpa E., Kelekis A., Galanos A., Vlahos L. Randomized, open label, prospective study on the effect of zoledronic acid on the prevention of bone metastases in patients with recurrent solid tumors that did not present with bone metastases at baseline. Med Oncol. 2005;22(2):195–201. doi: 10.1385/MO:22:2:195. [DOI] [PubMed] [Google Scholar]
- 69.Aft R., Naughton M., Trinkaus K., Watson M., Ylagan L., Chavez-MacGregor M. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 2010;11(5):421–428. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rack B., Juckstock J., Genss E.M., Schoberth A., Schindlbeck C., Strobl B. Effect of zoledronate on persisting isolated tumour cells in patients with early breast cancer. Anticancer Res. 2010;30(5):1807–1813. [PubMed] [Google Scholar]
- 71.Lin A.Y., Park J.W., Scott J., Melisko M., Goga A., Moasser M.M. Zoledronic acid as adjuvant therapy for women with early stage breast cancer and disseminated tumor cells in bone marrow. J Clin Oncol. 2008;26(S15):S559. [Google Scholar]
- 72.Vincenzi B., Santini D., Dicuonzo G., Battistoni F., Gavasci M., La Cesa A. Zoledronic acid-related angiogenesis modifications and survival in advanced breast cancer patients. J Interferon Cytokine Res. 2005;25(3):144–151. doi: 10.1089/jir.2005.25.144. [DOI] [PubMed] [Google Scholar]
- 73.Santini D., Vincenzi B., Hannon R.A., Brown J.E., Dicuonzo G., Angeletti S. Changes in bone resorption and vascular endothelial growth factor after a single zoledronic acid infusion in cancer patients with bone metastases from solid tumours. Oncol Rep. 2006;15(5):1351–1357. [PubMed] [Google Scholar]
- 74.Santini D., Vincenzi B., Galluzzo S., Battistoni F., Rocci L., Venditti O. Repeated intermittent low-dose therapy with zoledronic acid induces an early, sustained, and long-lasting decrease of peripheral vascular endothelial growth factor levels in cancer patients. Clin Cancer Res. 2007;13(15 Pt 1):4482–4486. doi: 10.1158/1078-0432.CCR-07-0551. [DOI] [PubMed] [Google Scholar]
- 75.Zhao X., Xu X., Guo L., Ragaz J., Guo H., Wu J. Biomarker alterations with metronomic use of low-dose zoledronic acid for breast cancer patients with bone metastases and potential clinical significance. Breast Cancer Res Treat. 2010;124(3):733–743. doi: 10.1007/s10549-010-1183-6. [DOI] [PubMed] [Google Scholar]
- 76.Ferretti G., Fabi A., Carlini P., Papaldo P., Cordiali Fei P., Di Cosimo S. Zoledronic-acid-induced circulating level modifications of angiogenic factors, metalloproteinases and proinflammatory cytokines in metastatic breast cancer patients. Oncology. 2005;69(1):35–43. doi: 10.1159/000087286. [DOI] [PubMed] [Google Scholar]
- 77.Tas F., Duranyildiz D., Oguz H., Camlica H., Yasasever V., Topuz E. Effect of zoledronic acid on serum angiogenic factors in patients with bone metastases. Med Oncol. 2008;25(3):346–349. doi: 10.1007/s12032-008-9043-5. [DOI] [PubMed] [Google Scholar]
- 78.Meraviglia S., Eberl M., Vermijlen D., Todaro M., Buccheri S., Cicero G. In vivo manipulation of Vgamma9Vdelta2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin Exp Immunol. 2010;161(2):290–297. doi: 10.1111/j.1365-2249.2010.04167.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sakamoto M., Nakajima J., Murakawa T., Fukami T., Yoshida Y., Murayama T. Adoptive immunotherapy for advanced non-small cell lung cancer using zoledronate-expanded gammadelta T cells: a phase I clinical study. J Immunother. 2011;34(2):202–211. doi: 10.1097/CJI.0b013e318207ecfb. [DOI] [PubMed] [Google Scholar]
- 80.Lang J.M., Kaikobad M.R., Wallace M., Staab M.J., Horvath D.L., Wilding G. Pilot trial of interleukin-2 and zoledronic acid to augment gammadelta T cells as treatment for patients with refractory renal cell carcinoma. Cancer Immunol Immunother. 2011;60(10):1447–1460. doi: 10.1007/s00262-011-1049-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gnant M., Mlineritsch B., Luschin-Ebengreuth G., Kainberger F., Kassmann H., Piswanger-Solkner J.C. Adjuvant endocrine therapy plus zoledronic acid in premenopausal women with early-stage breast cancer: 5-year follow-up of the ABCSG-12 bone-mineral density substudy. Lancet Oncol. 2008;9(9):840–849. doi: 10.1016/S1470-2045(08)70204-3. [DOI] [PubMed] [Google Scholar]
- 82.Brufsky A., Bundred N., Coleman R., Lambert-Falls R., Mena R., Hadji P. Integrated analysis of zoledronic acid for prevention of aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole. Oncologist. 2008;13(5):503–514. doi: 10.1634/theoncologist.2007-0206. [DOI] [PubMed] [Google Scholar]
- 83.Eidtmann H., de Boer R., Bundred N., Llombart-Cussac A., Davidson N., Neven P. Efficacy of zoledronic acid in postmenopausal women with early breast cancer receiving adjuvant letrozole: 36-month results of the ZO-FAST Study. Ann Oncol. 2010;21(11):2188–2194. doi: 10.1093/annonc/mdq217. [DOI] [PubMed] [Google Scholar]
- 84.De Boer R., Bundred N., Eidtmann H., Llombart A., Neven P., von Minckwitz G., et al. The effect of zoledronic acid on aromatase inhibitor-associated bone loss in postmenopausal women with early breast cancer receiving adjuvant letrozole: the ZO-FAST study 5-year final follow-up. In: Proceedings of the 33rd annual San Antonio breast cancer symposium; 2010. p. 8–12.
- 85.Coleman R.E., Thorpe H.C., Cameron D., Dodwell D., Burkinshaw R., Keane M., et al. Adjuvant treatment with zoledronic acid in stage II/III breast cancer. The AZURE trial (BIG 01/04)(abstract S4–S5). In: Proceedings from the San Antonio breast cancer symposium. San Antonio; 2010.
- 86.Gnant M. Zoledronic acid in breast cancer: latest findings and interpretations. Ther Adv Med Oncol. 2011;3(6):293–301. doi: 10.1177/1758834011420599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scagliotti G.V., Kosmidis P., de Marinis F., Schreurs A.J., Albert I., Engel-Riedel W. Zoledronic acid in patients with stage IIIA/B NSCLC: results of a randomized, phase III study. Ann Oncol. 2012;23(8):2082–2087. doi: 10.1093/annonc/mds128. [DOI] [PubMed] [Google Scholar]
- 88.Aft R.L., Naughton M., Trinkaus K., Weilbaecher K. Effect of (Neo)adjuvant zoledronic acid on disease-free and overall survival in clinical stage II/III breast cancer. Br J Cancer. 2012;107(1):7–11. doi: 10.1038/bjc.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Karim S.M., Brown J., Zekri J. Efficacy of bisphosphonates and other bone-targeted agents in metastatic bone disease from solid tumors other than breast and prostate cancers. Clin Adv Hematol Oncol. 2013;11(5):281–287. [PubMed] [Google Scholar]