INTRODUCTION
Endothelins (ETs) has a potent and sustained vasoconstrictive effect on a variety of blood vessels. The vascular smooth muscle cell (VSMC) is the target for ETs. VSMC of the whole body contains endothelin receptor (ETR)[1]. A great number of experiments have shown that three distinct complementary DNAs of ETR have been identified i.e., endothelin A receptor (ET-A receptor), endothelin B receptor (ET-B receptor) and endothelin C receptor (ET-C receptor). ET-A receptor was expressed in VSMC responsible for the contraction[2]. The aim of this study is to confirm the effects of endotoxin on the activity of ETR, and the transcription and expression of ET-A receptor mRNA in hepatic and intestinal tissues.
MATERIALS AND METHODS
Materials
Endotoxin (O111:B4) and porcine endothelin-1 (ET-1) were purchased from Sigma Chemical Co. 125I-ET-1 was supplied by Isotope Institute of Chinese Academy of Sciences. Digoxigenin DNA labeling and Detection Kit was provided by Boehringer Mannheim Co.
Methods
Thirty male and female Wistar rats weighing 210 g-215 g were fasted with access to water ad libitum for 24 h before experiment. Six rats were used as the control group. The others were given endotoxin at a dose of 10 mg/kg as an end otoxin-treated group. All rats were killed at the 3rd, 6th, 12th, and 24th hour after administration of endotoxin and saline, with 6 rats in each group at each time point. After sacrifice by decapitation at the respective time points, the right hepatic and intestinal tissues were immediately removed and preserved in liquid nitrogen for use.
The hepatic and intestinal plasma membrane was prepared by the method of Koseki and Imari[3] with minor modifications. The hepatic and intestinal tissue was homogenized in 10 vol (wt/vol) of 0.25 mol sucrose including 1 mmol EDTA, and the homogenate was centrifuged at 10000 × g for 10 min. The supernatant was centrifuged at 100000 × g for 1 h. The entire procedure was performed at 4 °C. The resulting pellet was resuspented in 200 μL of 0.25 mol/L sucrose including 1 mmol EDTA, and kept in the deep freezer for assay. Twenty μL aliquot of the microsomal fraction, containing 20 μg-protein in 0.25 mol sucrose including 1 mmol EDTA, was ke pt at 4 °C for 24 h, with various concentrations of 125I-ET-1 in 20 μL of the incubation solution. Twenty μL of ice-cold incubation solution was added and then centrifuged at 80000 × g at 4 °C for 30 min. The supernatant was rapidly aspirated. The radioactivity was determined with an FJ-2108 liquid scintillation counter. Specific binding was determined by the total binding minus the nonspecific binding. The maximal binding capacity (Bmax) and affinity (Kd) of ETR were obtained using Scatchard plot analysis.
Total cellular RNA was isolated from the hepatic and intestinal tissue with guanidinium thiocyanate-phenol-chloroform method[4]. Dot blot was used to identify and quantify ET-A receptor mRNA. Results were scanned in the computer to determine their quantity.
Statistical analysis
The data were expressed as mean ± SD. The difference between the groups was analyzed with Student′s t test. The difference was considered significant at P < 0.05.
RESULTS
The change of activity of hepatic and intestinal ETR
The values of the Bmax and Kd of ETR of the normal hepatic and intestinal tissue are shown in Figure 1, Figure 2. The Kd of ETR was 0.0328 in normal hepatic tissue, and 0.1263 in normal intestinal tissue. The Bmax of ETR was 1.0288 in normal hepatic tissue, and 1.2303 in normal intestinal tissue. The Bmax of ETR of the hepatic and intestinal tissue decreased gradually during endotoxemia. The Bmax of ETR in the endotoxin-treated group decreased more significantly than that in the control group at the 3rd hour after endotoxin administration (P < 0.01). The decrease of Bmax lasted 24 h. But the Kd of the hepatic and intestinal tissue did not change (Table 1).
Figure 1.
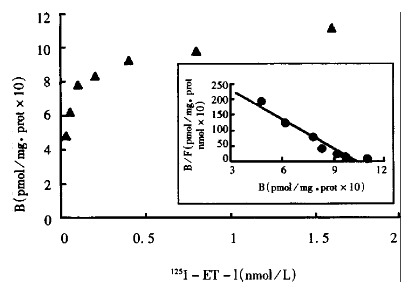
Specific binding of 125I-ET-1 on liver hemogenates and corresponding Scatchard plot for normal rats. KDa = 0.0328 nM, Bmax = 1.0288 pmol/mg
Figure 2.
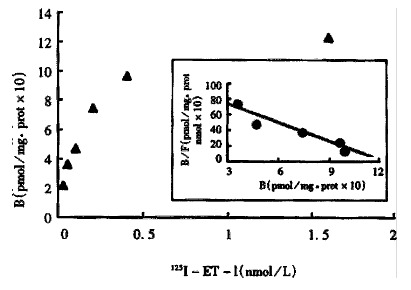
Specific binding of 125IET-1 on intestine hemogenates and corresponding Scatchard plot for normal rats. KDa = 0.1263 nM, Bmax = 1.2303 pmol/mg
Table 1.
Effects of endotoxin on ETR of hepatic and intestinal tissue
| Control |
Endotoxin-treated group |
||||
| 3rd | 6th | 12th | 24th (h) | ||
| Liver | |||||
| Bmax (bB/pmol•mg¯¹p) | 0.9969 ± 0.0155 | 0.7476 ± 0.0356b | 0.5590 ± 0.0219b | 0.4258 ± 0.0156b | 0.5826 ± 0.0586b |
| Kd (CB/nmol•L¯¹) | 0.0328 ± 0.0018 | 0.0338 ± 0.0016 | 0.0332 ± 0.0014 | 0.0324 ± 0.0010 | 0.0333 ± 0.0017 |
| Intestine | |||||
| Bmax (bB/pmol•mg¯¹p) | 1.2950 ± 0.2070 | 0.8810 ± 0.1026a | 0.6340 ± 0.2109b | 0.5199 ± 0.2260b | |
| Kd (CB/nmol•L¯¹) | 0.1309 ± 0.0183 | 0.1344 ± 0.0140 | 0.1181 ± 0.0173 | 0.1281 ± 0.0173 | 0.1260 ± 0.0169 |
P < 0.05,
P < 0.01 vs control group.
The change of the relative quantity of ETA receptor mRNA of hepatic and intestinal tissue
The relative quantity of ETA receptor mRNA of hepatic tissue in the endotoxin -treated group increased more significantly than that in the control group at 3-24 h (P < 0.01). It increased most obviously at the 6th and the 12th hour after endotoxin administration (Table 2). Compared with the control group, the relative quantity of ET-A receptor mRNA of intesti nal tissue in endotoxin-treated group also increased significantly between the 3rd and 12th hour after endotoxin administration (P < 0.01) (Table 2).
Table 2.
Effect of endotoxin on relative quantity of hepatic and inte stinal ET-A receptor nRNA (μg DNA/g RNA)
| Control |
Endotoxin-treated group |
||||
| 3rd | 6th | 12th | 24th (h) | ||
| Liver | 17.13 ± 2.11 | 57.96 ± 11.51b | 86.35 ± 16.01b | 95.50 ± 13.24b | 27.93 ± 3.05b |
| Intestine | 38.43 ± 3.86 | 48.32 ± 4.31b | 69.42 ± 8.21b | 68.61 ± 10.24b | 42.41 ± 3.73 |
P < 0.01 vs control group.
DISCUSSION
ETs have been shown to be one of the most potent vasoconstrictors, which are produced not only by vascular endothelial cells, but also by a variety of non-endothelial cells, and have many biological functions[5]. ETs should be combined with ETR of target cell, and activate a variety of signal transduction pathways so as to produce specific cellular responses[6]. This study showed that the Kd of ETR of hepatic and intestinal tissue did not change during endotoxemia. The Bmax of ETR of hepatic and intestinal tissue in endotoxin-treated group decreased more significantly in early stage than that in control group. Decrease of the Bmax of ETR lasted 24 h. The Bmax of ETR number of hepatic and intestinal tissue was the lowest at the 12th hour. After giving endotoxin, decrease of the ETR number of the hepatic and intestinal cell membrane was correlated with increase of ET-1 concentration (unpublished observation). Roubert et al[7] reported that the 50%receptors of VSMC in culture were induced by 10-9 mol ET-1. During endotoxemia, the higher level of ET-1 was, the more ET-1 combined with the receptor, the lower number of receptor of cell membrane was, because the receptors of cell membrane combining with ET-1 squeezed into cells.
The relative quantity of ETA receptor mRNA of hepatic and intestinal tissue increased obviously after endotoxin administration. This study showed that endotoxin could regulate transcription and translation of ETA receptor mRNA. The results of ETR binding assay and ETA receptor Dot blot analysis showed that the ETR number of hepatic and intestinal cell membrane decreased, but the relative quantity of ETA receptor mRNA of hepatic and intestinal tissue increased obviously in endotoxemia. It was likely that a mechanism of auto-regulation existed in the body. Down-regulation of ETR may be helpful in preventing endothelin from hyperconstraction and reducing damage of tissues and organs. So it suggested that ETR may play an important role in the hepatic and intestinal pathophysiologic process following endotoxemia.
Footnotes
Edited by Ma JY
References
- 1.Haynes WG, Webb DJ. The endothelin family of peptides: local hormones with diverse roles in health and disease问号 Clin Sci ( Lond) 1993;84:485–500. doi: 10.1042/cs0840485. [DOI] [PubMed] [Google Scholar]
- 2.Lüscher TF, Oemar BS, Boulanger CM, Hahn AW. Molecular and cellular biology of endothelin and its receptors--Part II. J Hypertens. 1993;11:121–126. doi: 10.1097/00004872-199302000-00002. [DOI] [PubMed] [Google Scholar]
- 3.Koseki C, Imai M, Hirata Y, Yanagisawa M, Masaki T. Autoradiographic distribution in rat tissues of binding sites for endothelin: a neuropeptide问号. Am J Physiol. 1989;256:R858–R866. doi: 10.1152/ajpregu.1989.256.4.R858. [DOI] [PubMed] [Google Scholar]
- 4.Cei WQ, Wang BY. Practical immunocytochemistry and nucleic acid hybridization techniques. Chengdu: Science andTechnology Press; 1994. pp. 406–429. [Google Scholar]
- 5.Masaki T, Kimura S, Yanagisawa M, Goto K. Molecular and cellular mechanism of endothelin regulation. Implications for vascular function. Circulation. 1991;84:1457–1468. doi: 10.1161/01.cir.84.4.1457. [DOI] [PubMed] [Google Scholar]
- 6.Kitsukawa Y, Gu ZF, Hildebrand P, Jensen RT. Gastric smooth muscle cells possess two classes of endothelin receptors but only one alters contraction. Am J Physiol. 1994;266:G713–G721. doi: 10.1152/ajpgi.1994.266.4.G713. [DOI] [PubMed] [Google Scholar]
- 7.Roubert P, Gillard V, Plas P, Chabrier PE, Braquet P. Down-regulation of endothelin binding sites in rat vascular smooth muscle cells. Am J Hypertens. 1990;3:310–312. doi: 10.1093/ajh/3.4.310. [DOI] [PubMed] [Google Scholar]


